Chapter: Modern Analytical Chemistry: Developing a Standard Method
Validating the Method as a Standard Method
Validating the
Method as a Standard Method
For an analytical method to be of use, it must be capable
of producing results with acceptable accuracy and
precision. The process
of verifying a method as de- scribed in the previous
section determines whether
the method meets
this goal for a single analyst. A further requirement for a standard
method is that an analysis should
not be affected by a change in the analyst
performing the work, the laboratory in which the work is performed, or the time when the analysis is conducted. The process by which a method is approved for
general use is known
as validation and involves a collaborative test of the method by analysts in sev-
eral laboratories. Collaborative testing is used
routinely by regulatory agencies and professional organizations, such as the U.S. Environmental Protection
Agency, the American Society for Testing
and Materials, the
Association of Offi- cial Analytical Chemists, and the American
Public Health Association, in estab- lishing their standard methods
of analysis.
When an analyst
performs a single
analysis on a sample, the difference be- tween the experimentally determined value and the expected value
is influenced by three sources
of error: random error, systematic errors inherent to the method, and systematic errors
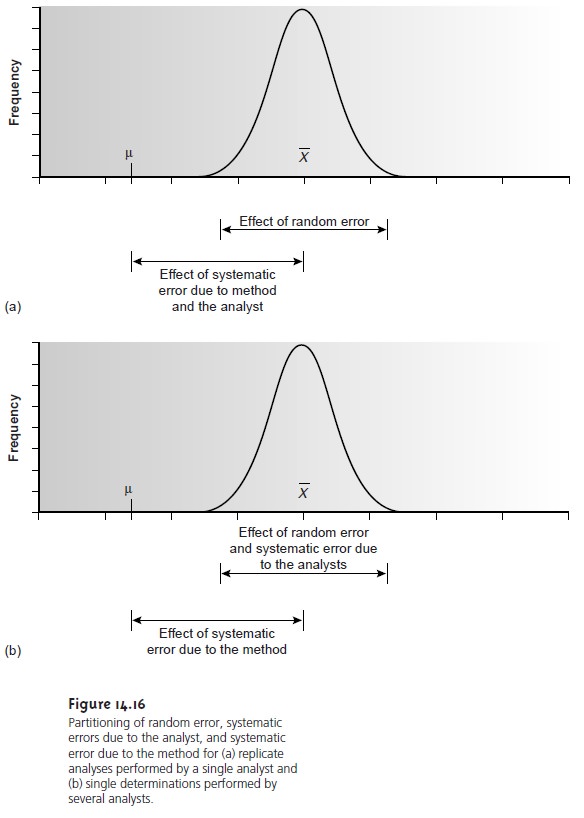
unique to the analyst. If enough replicate analyses are performed, a distribution of results can be plotted
(Figure 14.16a). The width
of this distribution is described by the standard
deviation and can be used to de- termine the effect of random error on the analysis. The position of the distribu- tion relative to the sample’s true value, μ, is determined both by systematic er- rors inherent
to the method and those systematic errors unique to the analyst. For a single analyst
there is no way to separate the total systematic error into its component parts.
The goal of a collaborative test is to determine the
expected magnitude of all
three sources of error when a method
is placed into general practice. When several analysts each analyze the same sample one time, the variation
in their col- lective results (Figure 14.16b) includes contributions from random errors and those systematic errors (biases) unique
to the analysts. Without additional infor-
mation, the standard
deviation for the pooled data cannot be used to separate
the precision of the analysis from the systematic errors of the
analysts. The posi- tion of the distribution, however, can be used to detect the presence of a system- atic error in the method.
Related Topics