Chapter: 10th Science : Chapter 9 : Solutions
Types of Solutions
Types
of Solutions
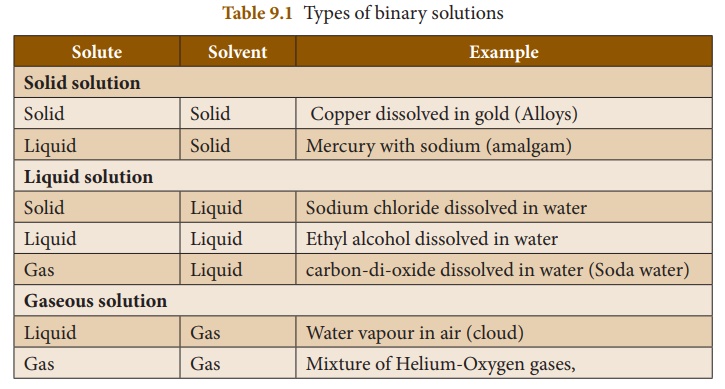
1. Based on the physical state of the solute and the solvent
We know that substances
normally exist in three physical states (phases) i.e., solid, liquid and gas.
In binary solutions, both the solvent and solute may exist in any of these
physical states. But the solvent constitutes the major part of the solution.
Its physical state is the primary factor which determine the characteristics of
the solution. Therefore, there are different types of binary solutions as
listed in Table 9.1.
2. Based on the type of solvent
Most of the substances
are soluble in water. That is why, water is called as ‘Universal solvent”.
However some substances do not dissolve in water. Therefore, other solvents
such as ethers, benzene, alcohols etc., are used to prepare a solution. On the
basis of type of solvent, solutions are classified into two types. They are
aqueous solutions and non-aqueous solutions.
a) Aqueous solution:
The solution in which
water acts as a solvent is called aqueous solution. In general, ionic compounds
are soluble in water and form aqueous solutions more readily than covalent
compounds. E.g. Common salt in water, Sugar in water, Copper sulphate in water
etc.
b) Non – Aqueous solution:
The solution in which
any liquid, other than water, acts as a solvent is called non-aqueous
solution. Solvent other than water is referred to as non-aqueous solvent.
Generally, alcohols, benzene, ethers, carbon disulphide, acetone, etc., are
used as non-aqueous solvents. Examples for non-aqueous solutions: Sulphur
dissolved in carbon disulphide, Iodine dissolved in carbon tetrachloride.

3. Based on the amount of solute
The amount of the solute
that can be dissolved in the given amount of solvent is limited under any given
conditions. Based on the amount of solute, in the given amount of solvent,
solutions are classified into the following types:
1.
Saturated solution
2.
Unsaturated solution
3.
Super saturated solution
1. Saturated solution: A solution in which
no more solute can be dissolved in a definite amount of the solvent at a given
temperature is called saturated solution. e.g. 36 g of sodium chloride in 100 g
of water at 25°C forms saturated solution.
Further addition of
sodium chloride, leave it undissolved.
2. Unsaturated solution:
Unsaturated solution
is one that contains less solute than that of the saturated solution at a given
temperature. e.g. 10 g or 20 g or 30 g of Sodium chloride in 100 g of water at
25°C forms an unsaturated solution.
3. Super saturated
solution: Supersaturated
solution is one that contains more solute than the saturated solution at a
given temperature. e.g. 40 g of sodium chloride in 100 g of water at 25°C forms
super saturated solution. This state can be achieved by altering any other
conditions liken temperature, pressure. Super saturated solutions are unstable,
and the solute is reappearing as crystals when the solution is disturbed.
4. Concentrated
and dilute solutions
It is another kind of
classification of unsaturated solutions. It expresses the relative
concentration of two solutions with respect to their solutes present in the
given amount of the solvent. For example, you are given two cups of tea. When
you taste them, you feel that one is sweeter than the other. What do you infer
from it? The tea which sweet more contains higher amount of sugar than the
other. How can you express your observation? You can say that the tea is
stronger. But a chemist would say that it is ‘concentrated’.
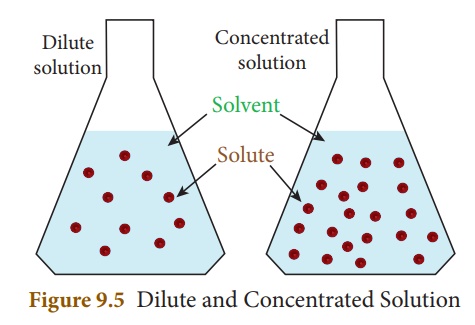
When we compare two
having same solute and solvent in a solutions, the one which contains higher
amount of solute per the given amount of solvent is said to be ‘concentrated
solution’ and the another is said to be ‘dilute solution’. They are
schematically represented by Figure 9.5.
Differentiating solutions
as dilute and concentrated is a qualitative representation. It does not imply
the quantity of the solute. This difference is observed by means of some
physical characteristics such as colour, density, etc.
Related Topics