Chapter: 10th Science : Chapter 9 : Solutions
Problems Based on Solubility and Percentage by Mass and Volume
Problems
Based on Solubility and Percentage by Mass and Volume
I. Problems based on solubility
1) 1.5 g of solute is
dissolved in 15 g of water to form a saturated solution at 298K. Find out the
solubility of the solute at the temperature.
Mass of
the solvent = 15 g
Solubility
of the solute = [ Mass of the solute/ Mass
of the solvent] × 100
Solubility
of the solute = [1.5/15] × 100
= 10 g
2) Find
the mass of potassium chloride would be needed to form a saturated solution in
60 g of water at 303 K? Given that solubility of the KCl is 37/100 g at this
temperature.
Mass of
potassium chloride in 100 g of water in saturated solution = 37 g
Mass of
potassium chloride in 60 g of water in saturated solution= 37/100 × 60
= 22.2 g
3) What
is the mass of sodium chloride that would be needed to form a saturated
solution in 50 g of water at 30°C. Solubility of sodium chloride is 36 g at
30°C?
At 30°C,
36 g of sodium chloride is dissolved in 100 g of water.
∴ Mass of
sodium chloride that would be need for 100 g of water = 36 g
∴ Mass of
sodium chloride dissolved in 50 g of water = [36 × 50] x 100
= 18 g
4) The
Solubility of sodium nitrate at 50°C and 30°C is 114 g and 96 g respectively.
Find the amount of salt that will be thrown out when a saturated solution of
sodium nitrate containing 50 g of water is cooled from 50°C to 30°C?
Amount of
sodium nitrate dissolved in 100 g of water at 50°C is 114 g
∴ Amount
of sodium nitrate dissolving in 50 g of
water at 50°C is = [114 × 50] / 100
= 57 g
Similarly
amount of sodium nitrate dissolving in 50g of water at 30°C is = [96 × 50] × 100
= 48g
Amount of
sodium nitrate thrown when 50g of water is cooled from 50°C to 30°C is
57 – 48 =
9 g
II. Problem based on mass percentage
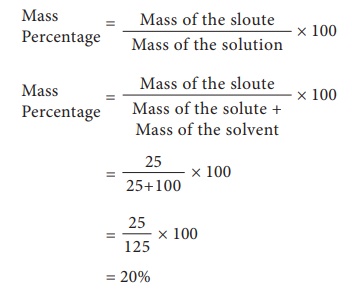
1) A
solution was prepared by dissolving 25 g of sugar in 100 g of water. Calculate
the mass percentage of solute.
Mass of
the solute = 25 g
Mass of
the solvent = 100 g
2) 16
grams of NaOH is dissolved in 100 grams of water at 25°C to form a saturated
solution. Find the mass percentage of solute and solvent.
Mass of
the solute (NaOH) = 16 g
Mass of
the solvent H2O = 100 g
(i) Mass percentage of the solute
Mass
percentage of solute = [Mass of the
solute / Mass of the solute + Mass of the solvent] × 100
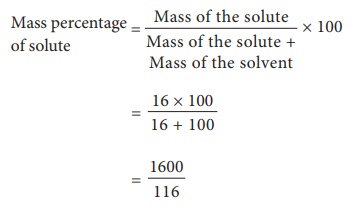
Mass
percentage of the solute = 13.79 %
(ii) Mass percentage of solvent
= 100 - (Mass percentage of the solute)
= 100 –
13.79 = 86.21%
3) Find
the amount of urea which is to be dissolved in water to get 500 g of 10% w/w
aqueous solution?
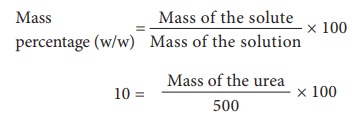
Mass of
urea = 50g
(iii) Problem based on Volume – volume percentage.
1) A
solution is made from 35 ml of Methanol and 65 ml of water. Calculate the
volume percentage.
Volume of
the ethanol = 35 ml
Volume of
the water = 65 ml
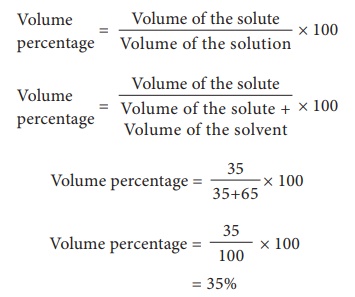
Volume
percentage = [ 35 / 35+65 ]× 100
Volume
percentage = [ 35/100 ] × 100
= 35%
2) Calculate
the volume of ethanol in 200 ml solution of 20% v/v aqueous solution of
ethanol.
Volume of
aqueous solution = 200 ml
Volume
percentage = 20%
Volume percentage
= [Volume of solute / Volume of solution]
× 100
20 = [Volume
of ethanol/200 ] × 100
Volume of
ethanol = [20 × 200] / 100 = 40 ml
Related Topics