Chapter: 10th Science : Chapter 9 : Solutions
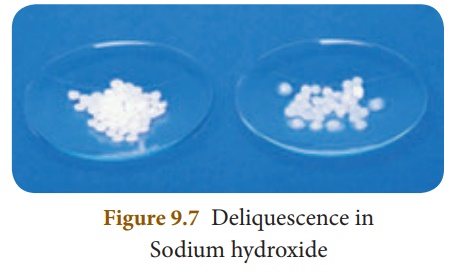
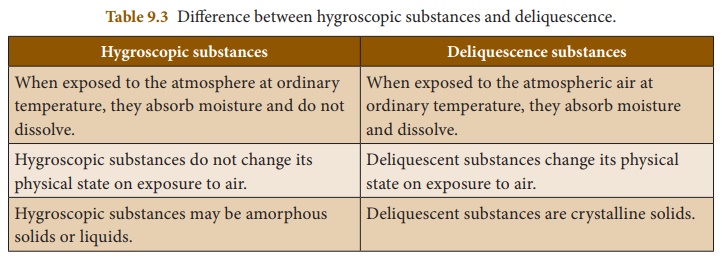
Deliquescence
Deliquescence
Certain substances which
are so hygroscopic, when exposed to the atmospheric air at ordinary
temperatures, absorb enough water and get completely dissolved. Such substances
are called deliquescent substances and this property is called deliquescence.
Deliquescent substances
lose their crystalline shape and ultimately dissolve in the absorbed water
forming a saturated solution.
Deliquescence is maximum
when:
1) The temperature is low
2) The atmosphere is
humid
Examples: Calcium chloride (CaCl2),
Caustic soda (NaOH), Caustic potash (KOH) and Ferric chloride (FeCl3).
Related Topics