Chapter: 10th Science : Chapter 9 : Solutions
Concentration of a Solution
Concentration
of a Solution
So far, we discussed
what is a solution? what does it consist of and its types. Most of the chemical
reactions take place in solutions form. So it is essential to quantify the solute
in solvent to study the reactions. To quantify the solute in a solution, we can
use the term “concentration”.
Concentration of a
solution may be defined as the amount of solute present in a given amount of
solution or solvent.
Quantitatively,
concentration of a solution may be expressed in different methods. But here, we
shall discuss percentage by mass (% mass) and percentage by volume (% volume).
1. Mass percentage
Mass percentage of a
solution is defined as the percentage by mass of the solute present in the
solution. It is mostly used when solute is solid and solvent is liquid.
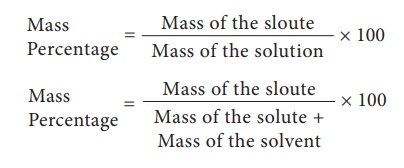
For example: 5% sugar
solution (by mass) means 5 g of sugar in 95 g of water. Hence it is made 100g
of solution.
Usually, mass percentage
is expressed as w/w (weight / weight); mass percentage is independent of
temperature.
2. Volume percentage
Volume percentage is
defined as the percentage by volume of solute (in ml) present in the given
volume of the solution.

For example, 10% by
volume of the solution of ethanol in water, means 10 ml of ethanol in 100 ml of
solution (or 90 ml of water)
Usually volume
percentage is expressed as v/v (volume / volume). It is used when both the
solute and solvent are liquids. Volume percentage decreases with increases in
temperature, because of expansion of liquid.
You can notice that in
the commercial products that we come across in our daily life such as a
solution of syrups, mouth wash, antiseptic solution, household disinfectants
etc., the concentration of the ingredients is expressed as v/v. Similarly, in
ointments, antacid, soaps, etc., the concentration of solutions are expressed
as w/w.
Related Topics