Solutions | Science - Points to Remember | 10th Science : Chapter 9 : Solutions
Chapter: 10th Science : Chapter 9 : Solutions
Points to Remember
Solutions (Science)
Points to Remember
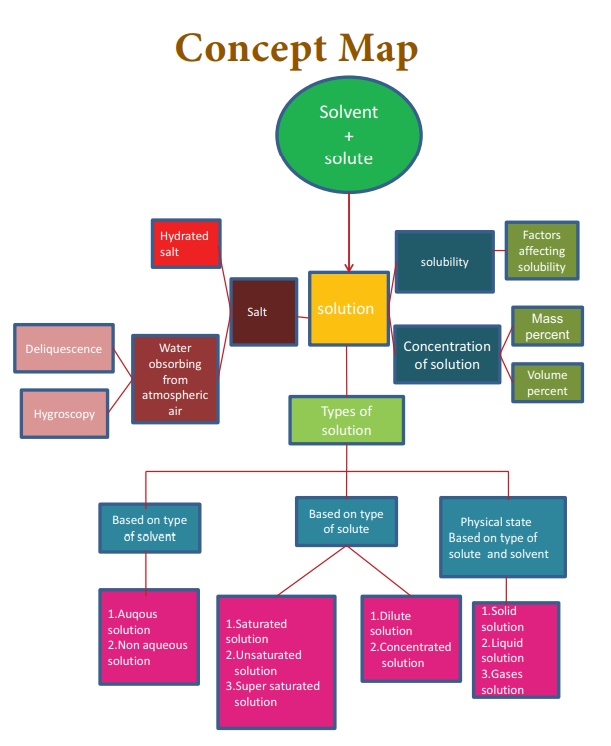
A solution is a homogeneous mixture of two or more substances.
An aqueous solution is a solution in which the solvent is water.
A non -aqueous solution is a solution in which the solvent is a liquid, other than water
A solution in which no more solute can be dissolved in a definite amount of the solvent at a given temperature is called saturated solution.
An unsaturated solution is one that contains less solute than the saturated solution at a given temperature.
A supersaturated solution is one that contains more solute than the saturated solution at a given temperature.
Polar compounds are soluble in polar solvents.
Non-polar compounds are soluble in non-polar solvents.
In endothermic process, solubility of solid solute increases with increase in temperature.
In exothermic process, solubility of solid solute decreases with increase in temperature.
Related Topics