Chapter: 10th Science : Chapter 9 : Solutions
Components of Solutions
COMPONENTS OF SOLUTIONS
We know that, a solution
is a homogeneous mixture of two or more substances. In a solution,
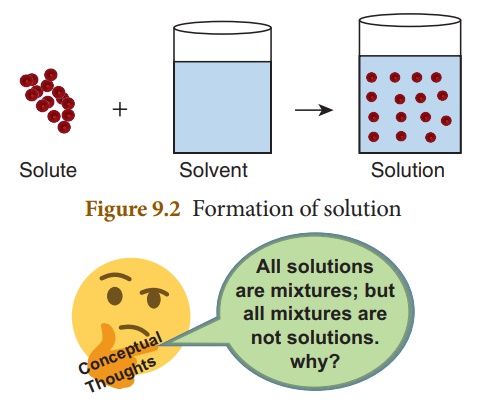
the component which is present in lesser amount (by weight), is called solute
and the component, which is present in a larger amount (by weight) is called solvent.
The solute gets distributed uniformly throughout the solvent and thus forming
the mixture homogeneous. So, the solvent acts as a dissolving medium in a
solution. The process of uniform distribution of solute into solvent is called dissolution.
Figure 9.2 shows the schematic representation of solution.
A solution must at least
be consisting of two components (a solute and a solvent). Such solutions which
are made of one solute and one solvent (two components) are called binary
solutions . e.g. On adding copper sulphate crystals to water, it
dissolves in water forming a solution of copper sulphate as shown in Figure
9.3. It contains two components i.e. one solute- copper sulphate and one
solvent-water. So it is a binary solution. Similarly, a solution may contain
more than two components. For example if salt and sugar are added to water,
both dissolve in water forming a solution. Here two solutes are dissolved in
one solvent. Such kind of solutions which contain three components are called ternary
solutions.

Related Topics