Introduction - Types of Chemical Reactions | 10th Science : Chapter 10 : Types of Chemical Reactions
Chapter: 10th Science : Chapter 10 : Types of Chemical Reactions
Types of Chemical Reactions
TYPES OF CHEMICAL REACTIONS
INTRODUCTION
As you know from your
earlier studies, a chemical reaction involves breaking of old chemical bonds
and formation of new chemical bonds. This change may happen spontaneously or it
may be facilitated by external forces or energy. Chemistry is all about
chemical reactions. In your day to day life, you could observe many chemical
reactions. A clear understanding of these reactions is essential in order to
manipulate them for the sake of human life and environment. So, chemistry
mainly focuses on chemical reactions. Let us try to find the answer for the
following questions:
·
You need energy to play, walk, run or to perform various physical
activities. Where do you get the energy from?
·
How do plants grow and get their food? How does a car move using
fuel?
·
Why does iron rust on its exposure to water or air?
You get energy from the
digestion of the food you eat. Plants grow by absorbing nutrients from the
Earth and get their food by photosynthesis. The combustion of a fuel makes the
car to move. Oxidation of iron causes rusting. So, all these processes are
chemical changes i.e. the materials, which undergo changes are converted into
some other new materials. For example, by burning petrol, the hydrocarbons
present in it are converted into carbon dioxide and water. In this chapter, let
us discuss the nature and types of chemical reactions.
What happens during a chemical reaction?
·
In a chemical reaction, the atoms of the reacting molecules or
elements are rearranged to form new molecules.
·
Old chemical bonds between atoms are broken and new chemical bonds
are formed.
·
Bond breaking absorbs energy whereas bond formation releases
energy
How are chemical reactions represented?
When methane reacts with
oxygen, it forms carbon dioxide and water. How can you represent this reaction?
It can be written as a word equation as shown below:
Methane + Oxygen →
Carbon dioxide + Water
But, this equation does
not give the chemical composition of the reactants and products. So, to learn
the characteristics of a chemical reaction, it is represented by a chemical
equation. In the chemical equation, the chemicals of the reaction are
represented by their chemical formulas.The compounds or elements, which undergo
reactions (reactants) are shown to the left of an arrow and the compounds
formed (products) are shown to the right of the arrow. The arrow indicates the
direction of the reaction. Thus, the aforesaid reaction can be written as
follows:
CH4 + O2
→ CO2 + H2O
But, this is also an
incomplete chemical equation. Because, the law of conservation of matter states
that matter cannot be created or destroyed. You cannot create new atoms by a
chemical reaction. In contrast, they are rearranged in different ways by a
chemical reaction to form a new compound. So, in a chemical equation, the
number of atoms of the reactants and that of the products must be equal. The
number of hydrogen and oxygen atoms in the reactants and the products are not
equal in the given equation. On balancing the number of atoms, the following
equation can be obtained:![]()
![]()
CH4 + 2O2
→ CO2 + 2H2O
Further, the chemical
equation provides information on the physical state of the substances and the
conditions under which the reaction takes place.
CH4(g) + 2O2(g)
→ CO2(g) + 2H2O(g)
Methane Oxygen Carbon
dioxide Water
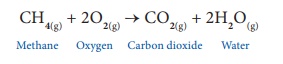
A balanced chemical equation is the simplified representation of a
chemical reaction which describes the chemical composition, physical state of
the reactants and the products, and the reaction conditions.
Related Topics