Types of Chemical Reactions - Points to Remember | 10th Science : Chapter 10 : Types of Chemical Reactions
Chapter: 10th Science : Chapter 10 : Types of Chemical Reactions
Points to Remember
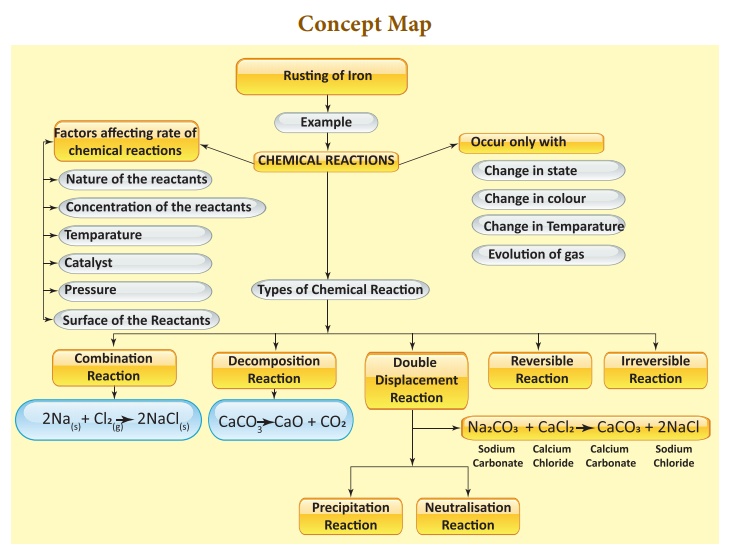
Types of Chemical Reactions
Points to Remember
· A chemical change is a change in which one or more new substances are formed.
· Aerobic: Presence of oxygen.
· Anaerobic: Absence of oxygen
· Most combination reactions are exothermic
· Electrolytic decomposition reaction may occur in the presence of heat or light.
· All photo decomposition reaction are endothermic reactions.
· Double displacement reaction or
· metathesis may occur by the mutal
· exchange of ions.
· Precipitation reaction gives an insoluble salt as the product.
· Neutralisation reactions are reactions between an acid and a base that forms salt and water.
· Plants can not grow in an acidic soil. Neutralisation prevents tooth decay.
· Most reactions in chemistry are irreversible reactions.
· Chemical equilibrium-the rate of the forward reaction is equal to rate of the back ward reactions.
· Equilibrium is possible in a closed system. Temperature increases the reaction rate. Pressure increases the reaction rate.
· The term pH means power of hydrogen. pH plays a vital role in everyday life.
· In humans all bio chemical reactions take place between the pH value of 7.0 to 7.8.
· If pH of rain water is below 5.6 its called acid rain.
· Pure water is a weak electrolyte.
Related Topics