Chapter: 10th Science : Chapter 10 : Types of Chemical Reactions
pH Scale
pH SCALE
All the aqueous
solutions may contain hydrogen and hydroxyl ions due to self-ionisation of
water. In addition to this ionisation, substances dissolved in water also may
produce hydrogen ions or hydroxyl ions. The concentration of these ions decides
whether the solution is acidic or basic. pH scale is a scale for measuring the
hydrogen ion concentration in a solution. The 'p' in pH stands for ‘Potenz’ in
German meaning 'power'. pH notation was devised by the Danish biochemist
Sorensen in 1909. pH scale is a set of numbers from 0 to 14 which is used to
indicate whether a solution is acidic, basic or neutral.
·
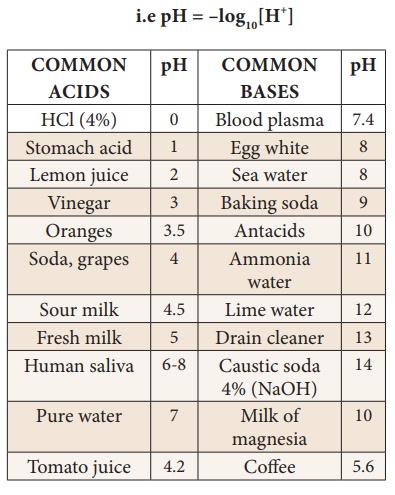
Acids have pH less than 7
·
Bases have pH greater than 7
·
A neutral solution has pH equal to 7
The pH is the negative
logarithm of the hydrogen ion concentration
i.e pH = –log10[H+]
How can we measure the
pH of a given solution using pH Paper
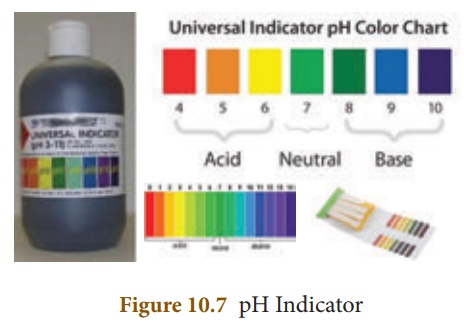
The pH of a solution can
be determined by using a universal indicator. It contains a mixture of dyes. It
comes in the form of a solution or a pH paper.
A more common method of
measuring pH in a school laboratory is by using the pH paper. A pH paper
contains a mixture of indicators. It shows a specific colour at a given pH. A
colour guide is provided with the bottle of the indicator or the strips of paper
impregnated with it, which are called pH paper strips. The test solution is
tested with a drop of the universal indicator, or a drop of the test solution
is put on the pH paper. The colour of the solution on the pH paper is compared
with the colour chart and the pH value is read from it. The pH values thus
obtained are only approximate values.
Related Topics