Chapter: Medical Immunology: Tolerance and Autoimmunity
Treatment of Autoimmune Diseases
TREATMENT OF AUTOIMMUNE DISEASES
Standard therapeutic approaches to autoimmune disease usually involve symptomatic pal-liation with anti-inflammatory drugs and attempts to downregulate the immune response. Glucocorticoids, which have both anti-inflammatory and immunosuppressive effects, have been widely used, as have immunosuppressive and cytotoxic drugs. However, the use of these drugs is often associated with severe side effects and is not always efficient. Other therapeutic approaches that have been tried have had as their objective inducing tolerance or at least downregulating the autoimmune response.
Induction of tolerance to the responsible antigen is the most logical approach to the treatment of autoimmune disorders. This approach is hampered by the fact that the identity of the antigen is not known with certainty in many diseases. However, this may not be an insumountable obstacle, due to the phenomenon recently described as “bystander toler-ance.” When a cross-reactive antigen is used to induce oral tolerance, immunoregulatory cells secreting IL-10 and TGF-β differentiate in the submucosa and migrate to lymphoid organs and inflamed site, where they suppress the activity of pro-inflammatory TH1 cells. The effects of regulatory cells are not antigen-specific, so they may extend to autoreactive T cells interacting with a peptide different from those generated by the orally-administered tolerogen. Examples of the beneficial effects of oral tolerization have been described, both in animal models and humans. In experimental animals, oral administration of basic myelin protein has been shown to decrease the severity of experimental allergic encephalitis. In pa-tients with rheumatoid arthritis oral administration of collagen type II was followed by clin-ical improvement.
B-cell tolerization has been successfully tried in patients with SLE. Administration of a construct of four short DNA fragments conjugated to a dextran backbone caused ces-sation of DNA antibody synthesis. Apparently, the construct bound to B-cell surface im-munoglobulins in DNA-specific B cells and caused its internalization. The reason why this causes the interruption of antibody synthesis has not been clarified.
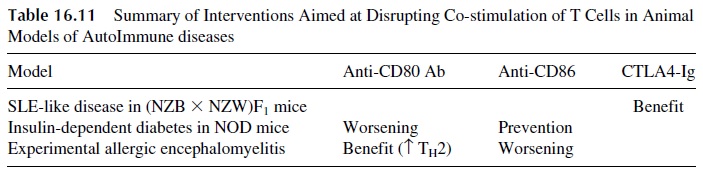
The knowledge that co-stimulatory signals are essential for T-cell activation has led to attempts to induce anergy by disrupting co-stimulatory interactions, with variable but promising results in animal models (see Table 16.11). Some interesting results have been obtained in mice that develop SLE-like disease:
· Administration of anti-CD40L monoclonal antibodies to asymptomatic (NZB×NZW) F1 female mice delays the onset of nephritis. This approach seems to to-lerize B cells, which otherwise would be involved in the synthesis of DNA au-toantibodies, and is believed to play a critical role in the development of glomerulonephritis in SLE .
· Administration of anti-CD40 monoclonal antibodies can induce the reversion of es-tablished nephritis in (NZB×NZW) F1female mice, possibly by blocking the interaction between activated autoreactive T cells and CD40-expressing en-dothelial cells. In the absence of this co-stimulatory signal the autoreactive T cells may become downregulated and the disease progress is blocked.
Disruption of the action of cytokines using soluble forms of cytokine receptors has been extensively considered. A recombinant, soluble TNF receptor produced by a hybrid genome in which the TNF receptor gene was fused to a human IgG Fc gene has been suc-cessfully introduced in the treatment of rheumatoid arthritis. In this case, the addition of the IgG constant region gene has the effect of prolonging the half-life of the recombinant TNF receptor in circulation. Similarly, CTLA4Ig (fusion of CTLA4 to Fc IgG) has been used in the treatment of murine lupus, and it was recently reported to have therapeutic effects in pa-tients with psoriasis.
Injection of normal pooled immunoglobulins (IVIg) has been tried in a number of hu-man autoimmune diseases and proved to be of definite help in a form of pediatric vasculi-tis (Kawasaki’s syndrome) as well as in many cases of idiopathic thrombocytopenic pur-pura. The mechanism of action is believed to involve B-cell downregulation as a consequence of the simultaneous cross-linking of membrane immunoglobulins by anti-id-iotypic antibodies contained in the IVIg preparations and of Fc receptors. The consequence of this downregulation is a decreased synthesis of auto-antibodies.
Elimination or downregulation of T cells by injection of monoclonal anti–T-cell an-tibodies has been shown to be therapeutic in a number of animal models as well as in hu-man transplantation. Murine monoclonal antibodies are immunogenic and unsuitable for long-term use in humans. However, it is possible that genetically engineered humanized monoclonal antibodies will prove useful. These humanized monoclonals are encoded by re-combinant genomes in which all immunoglobulin-coding genes minus those coding for the antibody-binding site are of human origin, while the genes coding for the specific antibody binding site are obtained from a murine B-cell clone of known specificity. Because the im-munogenic epitopes are predominantly located in the constant regions, which in these mon-oclonals are homologous, humanized monoclonals can be repeatedly administered to hu-mans with low risk of inducing serum sickness. The clinical value of these antibodies in human autoimmune diseases is being evaluated.
Administration of immunotoxins that have been prepared by combining either mon-oclonal antibodies or IL-2 with cytotoxins is expected to increase the destruction rate of the cells responsible for the autoimmune process. These approaches have not met with great success. It is hoped that better definition of cell markers for activated lymphocyte subsets involved in the pathogenesis of autoimmune diseases may lead to the introduction of more specific and more effective antibodies or immunotoxins.
Reestablishment of a perturbed TH1/TH2 lymphokine balance has been successful in a number of animal models. TH 2 diseases (such as lupus) would benefit from blockade of the action of TH 2 cytokines such as IL-4, whereas TH1 diseases (such as EAE) would ben-efit form the administration of IL-4. However, the effects of cytokines are pleiotropic, and the expected outcome of these interventions in humans may not be accomplished without undesirable side effects.
Autoimmune cells have been shown to display signaling abnormalities, and it has been suggested that use of drugs that block certain kinase activity to be of therapeutic value. It has been reported that administration of tyrphostin, an inhibitor of Janus-associated ki-nase (JNK), reduced EAE disease activity.
Autologous hematopoietic cell transplantation following ablation of the immune sys-tem has been reported to be helpful in patients with systemic autoimmune diseases that are refractory to conventional treatment. Current multicenter trials will determine the useful-ness of this modality in the treatment of various severe autoimmune disorders including rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus. It is expected that the eradication of the immune system and grafting of normal stem cells will set the im-mune clock back several years.
Plasmapheresis consists of pumping the patient’s blood through a special centrifuge to separate plasma from white and red cells. The red cells and plasma substitutes are pumped back into the patient, while the plasma is discarded. The rationale for plasma-pheresis in the treatment of autoimmune diseases is to remove pathogenic autoantibodies and immune complexes from the circulation.
Related Topics