Chapter: Modern Medical Toxicology: Substance Abuse: Substances of Dependence and Abuse
Tobacco - Substances of Dependence and Abuse
Tobacco
Sources
·
Nicotiana attenuata (Wild tobacco)
·
Nicotiana glauca (Tree tobacco)
·
Nicotiana longiflora (Cultivated ornamental)
·
Nicotiana rustica
·
Nicotiana tabacum (Commercial tobacco)
· Nicotiana trigonophylla (Desert tobacco).
Tobacco is usually prepared from
cured leaves of Nicotianatabacum (Fig 34.1) belonging to family
Solanaceae. Turkishtobacco is prepared from the leaves of Nicotiana rustica, and is more potent. Indian tobacco refers to Lobelia inflata.

Active Principles
·
Nicotiana tabacum and N.rustica contain the following alka-loids:
·
Nicotine
·
Nornicotine
·
Anabasine
·
Anabatine.
Nicotiana tabacum is an annual herb, shrub, or small
tree; from0.90 to 1.50 metres tall according to the variety. The leaves are
elliptical or oblanceolate; flowers are clustered at the end of the branches
and have a cylindrical calyx, being greenish or reddish in the upper part (Fig 34.2). Fruit has different forms
with globular seeds. Every part of the plant (except the seed) contains
nicotine, the maximum concentration of which is in the leaves. Lobelia inflata contains lobeline. It is
sometimes used as a nicotine substitute. Nicotine is a colourless to pale
yellow, very hygroscopic, oily liquid with an unpleasant pungent odour, and
sharp, burning, persistent taste. It gradually becomes brown on exposure to air
or light.

Uses
·
Nicotine is a stimulant of the central nervous system, and
is abused widely all over the world in the form of inhalation (ciga-rette,
cigar, pipe, beedi), nasal insufflation (snuff), or chewing.
·
Nicotine is also used as an insecticide.
Mode of Action
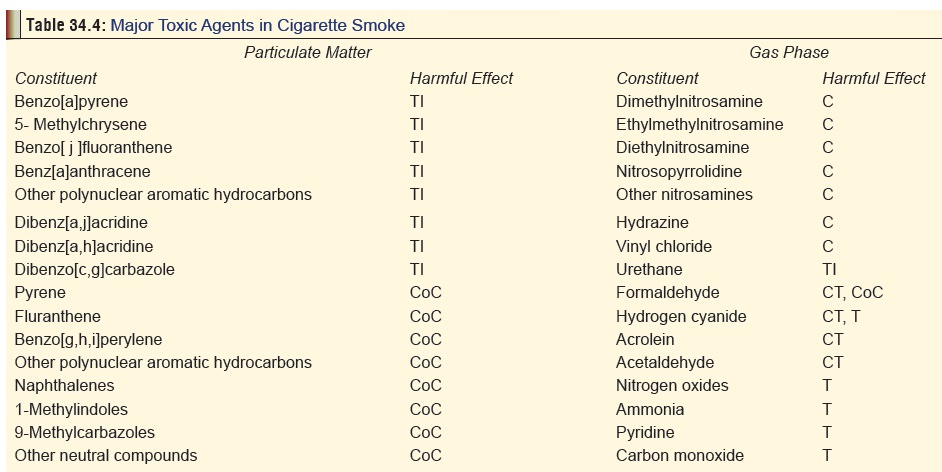
By far the commonest source of nicotine poisoning (acute or chronic) results from smoking tobacco in the form of cigarettes. When a cigarette is lit and inhaled, the smoker is exposed to both gaseous and particulate matter. Nicotine and tar are part of the particulate phase of cigarette smoke (Table 34.4 ). The usual nicotine content of a “regular” ciga-rette varies between 13 and 20 mg, while certain European and Turkish cigarettes can contain higher amounts. “Low nicotine” cigarettes contain 7 to 10 mg of the alkaloid. Cigars contain 15 to 40 mg of nicotine. When a cigarette is smoked, more than half the nicotine escapes in the sidestream smoke, while a large fraction remains in the butt and filter, and it is only 0.5 to 2 mg (average 1 mg) of nicotine that finally is delivered to the smoker. Smoke from non-filtered cigarettes contains slightly higher amounts of nicotine. This amount depends not only on the nicotine content of the cigarette, but also on the individual’s smoking technique (rate of puffing, puff volume, depth of inhalation, and size of residual butt).

In India, “bidis” (Fig 34.3 ) are very popular, especially
among the poorer sections of society, since they are much cheaper than
cigarettes. Bidis are small, brown, hand-rolled cigarettes consisting of
tobacco wrapped in a tendu or temburni leaf (Diospyros melanoxylon) (Fig
34.4). They are more harmful than cigarettes and produce higher levels of
carbon monoxide, nicotine, and tar. Also, because of low combusti-bility of
tendu leaf, bidi smokers tend to inhale more often and more deeply, breathing
in greater quantities of tar and other toxins than cigarette smokers.


After cigarettes, the next common
source of nicotine toxicity results from smokeless tobacco which is of two
kinds snuff and chewing tobacco. There has been a resurgence of popularity in
the use of snuff in recent times, paralleling the decline in cigarette smoking
in most parts of the world. Because smoking is not involved, people generally
believe that snuff is more socially acceptable and less harmful. This is
however not true. Snuff is usually available as finely cut tobacco powder which
is packaged dry or moist. It contains approximately 14 mg of nicotine per gram
of tobacco.
Chewing tobacco is generally packaged as “twists” (leaf tobacco twisted into rope-like portions) (Fig 34.5) or “plugs” (shredded tobacco pressed into cakes) (Fig 34.6) . These are chewed or simply placed between the cheek and gums. The nicotine dissolves in the saliva and is absorbed through the mucous membrane of the mouth, as well as through the intes-tinal mucosa after the saliva is swallowed. A portion of the tobacco that is placed in the mouth each time for chewing is referred to as a “quid”. A typical bite-size quid contains 1.5 to 2.5 grams of tobacco. Ultimately, the tobacco chewer gets approximately the same dose of nicotine (or slightly more) than the tobacco snuffer. The smokeless tobacco user who takes 8 to 10 quids per day gets a nicotine equivalent of 30 to 40 cigarettes per day.


Though nicotine insecticides have
been banned in most parts of the world since 1950, they are still available in
some formulations. Several cases of severe nicotine poisoning due to exposure
(dermal and oral) to these pesticides have been reported, some of which have
ended in death.
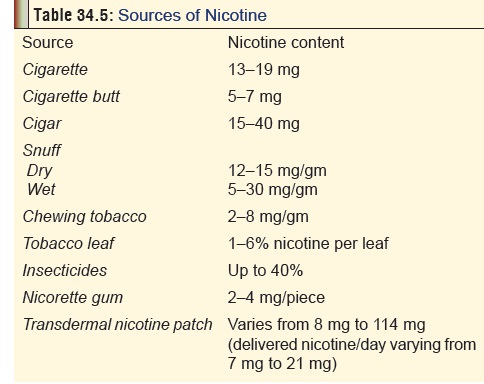
The nicotine content of all these
sources has been summarised in Table
34.5.
Mode of Action
·
Nicotine binds stereo-specifically to select acetylcholine
receptors (nicotine receptors). These receptors are present throughout the
body, particularly in the autonomic ganglia, adrenal medulla, central nervous
system, spinal cord, neuromuscular junctions, and chemoreceptors of carotid and
aortic bodies. In the CNS, the highest concentration of nicotine receptors is
found in the limbic system, midbrain, and brainstem.
· At moderate doses, nicotine stimulates the reticular acti-vating system producing an alerting pattern on the EEG, with resultant favourable effects on memory and attention.
·
But higher doses cause tremor and convulsions due to a CNS
disinhibition mechanism.
·
Nicotine stimulation of vagal centres in the medulla induces
nausea and vomiting, while the gastro-oesophagal reflux is provoked due to a
lowering of sphincter pressure and increased acid secretion. Larger doses cause
diarrhoea due to both central and parasympathetic excitation.
·
By acting directly on nicotine receptors in endocrine
glands, as well as by stimulating neurohumoral pathways in the CNS, nicotine
enhances release of catecholamines, vaso-pressin or antidiuretic hormone,
growth hormone, ACTH, cortisol, prolactin, serotonin, and beta endorphins.
Nicotine also increases amylase, trypsin and chymotrypsin activity.
·
Nicotine suppresses appetite while increasing basal energy
expenditure, resulting in weight loss.
·
Habitual use of nicotine by women results in decreased
oestrogen levels (due to enhanced hydroxylation of oestra-diol), thereby
increasing the risk of osteoporosis.
The physiological effects of
nicotine are summarised in Table 34.6.

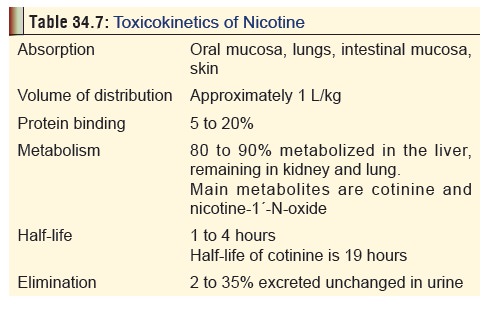
Toxicokinetics
Mentioned
in Table 34.7.
Drug Interactions
Smoking alters the metabolism of
some commonly used drugs. Metabolism is enhanced in the case of
benzodiazepines, caffeine, H2 antagonists, imipramine, nicotine,
opiates, phen-acetin, propranolol, and theophylline. As a result of such
inter-ference, the therapeutic efficacy of opiates, benzodiazepines,
beta-adrenergic antagonists, nifedipine, H2 antagonists, and
antacids is reduced. This alteration of drug metabolism is due to induction of
microsomal enzyme systems, not by nicotine itself, but most probably by
polynuclear aromatic hydrocarbons. Drugs using the P450 system are not affected
by smoking.![]()
While smoking has no effect on the
clearance of alcohol, concomitant use exaggerates the cardiovascular response
of nicotine, as a result of which the heart rate and blood pressure go up. This
is thought to be catecholamine mediated, and it has been suggested that smokers
may have increased tendency to suffer from arrhythmias and sudden death during
alcohol use.
Clinical (Toxic) Features
1. Acute Poisoning:
a. Early Effects (15min to 1 hour)—
––
GIT: Nausea, salivation, vomiting, abdominal pain.
––
CVS: Tachycardia, hypertension.
––
RS: Tachypnoea, bronchorrhoea.
–– CNS: Agitation, anxiety,
sweating, headache, blurred vision, confusion, vertigo, tremor, ataxia, muscle
fasciculations, convulsions. Pupils are at first constricted, but may dilate
later. A primary position upbeat nystagmus is seen following ciga-rette
smoking, chewing of nicotine gum, and inges-tion of nicotiana glauca leaves, and is the direct result of nicotine.
b. Delayed Effects (after1 hour)— –– GIT: Diarrhoea.
–– CVS: Bradycardia, arrhythmias,
hypotension, shock.
––
RS: Hypoventilation, apnoea.
–– CNS: lethargy, weakness,
hyporeflexia, hypotonia, paralysis, coma.
Death may occur, especially in the
case of ingestion of cigarettes (inadvertently) by children, or exposure to
insec-ticidal nicotine. Nicotine ingestion causes hypertension and tachycardia,
followed by hypotension and bradycardia, headache, CNS stimulation followed by
depression, tremors and seizures, hallucinations, confusion, hyperpnoea, mucous
membrane irritation, and vomiting. Ingestion of large amount can cause weakness,
paralysis, coma, and rarely respiratory failure resulting in death.
Occupational dermal exposure to wet,
uncured tobacco may produce “green tobacco sickness” among workers,
char-acterised by nausea, vomiting, headache, vertigo, pallor, and sweating.
Headache occurs frequently, especially in harvesters. Contact dermatitis can
occur. Harvesters who are smokers are resistant to most of these effects.
2. Chronic Poisoning (Addiction):
Nicotine dependence is the most
widely preva - lent and deadly of all substance dependencies. DSM -IV defines
two nicotine-related disorders: nicotine dependence and nicotine withdrawal.
Nicotine abuse is not included in DSM-IV, but a related term “harmful use” is
mentioned in ICD -10, which means that continued use causes physical problems.
The dependence-producing effects of nicotine appear to be modu-lated by
dopamine which is increased in smokers. Nicotine also increases noradrenaline,
adrenaline, and serotonin levels. Like ![]() most substance use, nicotine use
begins because of social rein-forcement. With repeated exposure, many
youngsters find the physiological effects of nicotine well suited to help them
with the difficult periods during adolescence. In addition, physical dependence
begins so that cessation of nicotine use becomes uncomfortable. Children more
likely to start smoking are those who have a high need to conform, display low
academic perfor-mance, rebelliousness, depressive symptoms, and have poor
self-esteem. Peer and family influences also play a major role.
most substance use, nicotine use
begins because of social rein-forcement. With repeated exposure, many
youngsters find the physiological effects of nicotine well suited to help them
with the difficult periods during adolescence. In addition, physical dependence
begins so that cessation of nicotine use becomes uncomfortable. Children more
likely to start smoking are those who have a high need to conform, display low
academic perfor-mance, rebelliousness, depressive symptoms, and have poor
self-esteem. Peer and family influences also play a major role.
a. Health consequences of tobacco use: –– Lung cancer.
––
Non-pulmonary cancers: Mouth, larynx, oesoph-agus, stomach, liver, pancreas,
bladder, uterine cervix, breast, brain.
––
Respiratory diseases: Emphysema, bronchitis, asthma, pneumonia.
––
Cardiovascular diseases: Coronary heart disease, hypertension, arterial
thrombosis, stroke.
––
Obstetric and neonatal conditions: Abortion, abruptio placenta, placenta
praevia, preterm labour, pre-eclampsia, growth retardation, congenital
malformations, sudden infant death syndrome, foetal or neonatal death.
––
Other conditions: Peptic ulcer, osteoporosis, Alzheimer’s disease.
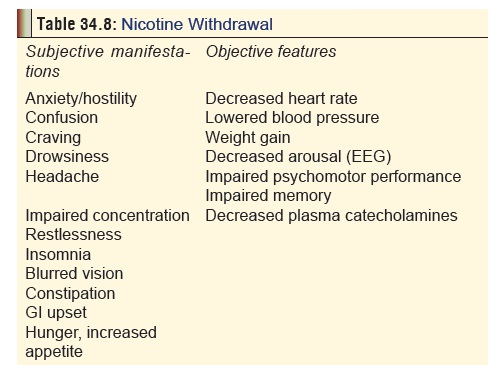
b. Nicotine withdrawal: Manifestations of nicotine with-drawal can occur within 4 to 8 hours of the last ciga-rette. In fact most chronic smokers experience some withdrawal symptoms on waking up each morning. Manifestations include changes in mood, insomnia, difficulty concentrating, restlessness, decreased heart rate (average decline is 8 beats per minute), and weight gain (average is 2 to 3 kg). Craving is common, and increased coughing, poor performance on vigilance tasks, etc., can occur. Clinical manifestations of nicotine withdrawal are summarised in Table 34.8. Nicotine withdrawal is worst in cigarette smokers, intermediate in users of smokeless tobacco, and mild in users of nicotine replacement products. The syndrome peaks in 1 to 3 days and lasts for 3 to 4 weeks. In some, it may last for several months, especially features such as craving and weight gain.
Usual Fatal Dose
Nicotine
is highly toxic; 2 to 5 mg can cause nausea, and 40 to 60 mg can cause death.
However, survival has occurred with ingestions of 1 to 4 grams.
Diagnosis
·
Acute poisoning can be confirmed by estimating plasma
nico-tine level; but the short half-life of nicotine necessitates early
withdrawal of blood. High pressure liquid chromatography is generally utilised
to assay nicotine levels. Plasma level greater than 40 to 50 ng/ml indicates
serious toxicity.
·
Polymorphonuclear leucocytosis and glycosuria are often
encountered in nicotine overdose.
·
Passive tobacco smoke exposure is usually determined by
estimating cotinine levels in plasma, urine, or saliva. Urine cotinine is also
used as an index to nicotine exposure in tobacco workers (especially
harvesters).
Treatment
1. Acute Poisoning
Mild
overdose (with spontaneous vomiting) requires only observation for 4 to 6
hours, after which the patient can be discharged. Serious overdose must be
treated as follows:
·
Decontamination by stomach wash.
Emesis is contrain-dicated. Activated charcoal is effective and must be
administered in the usual manner. In cases of dermal exposure (e.g. wet tobacco
leaves, spillage of nicotine liquid), clothing should be removed, and skin
thor-oughly washed.
·
Since nicotine is weakly alkaline,
excretion can be enhanced by acidification of urine. But it is not recom-mended
by most investigators since it can aggravate the condition of a convulsing
patient in whom there is rhabdomyolysis.
·
Animal experiments indicate that
drugs such as pempidine and mecamylamine may have antidotal effects against
nicotine. Hexamethonium (a ganglionic blocking agent) has prevented
nicotine-induced convul-sions in animals.
Symptomatic
and supportive measures—
––
Benzodiazepines for convulsions.
–– Atropine for bradycardia.
––
IV fluids and vasopressors (dopamine or noradrena-line) for hypotension.
––
Respiratory compromise is managed by oxygen, intubation, and positive pressure
ventilation.
2. Chronic Poisoning (Addiction)
Nicotine withdrawal must be treated
by a combination of therapies including psychosocial, psychopharmacological,
and nicotine replacement. A psychiatrist’s help is crucial to effective
management of withdrawal and prevention of relapse.
o Nicotine replacement therapy—
The rationale behind nicotine
replacement is to prevent or relieve nicotine withdrawal symptoms while
stop-ping smoking behaviour by replacing it with another behaviour.
Pharmacological nicotine is of various forms and dosages.
–– Nicotine gum (Polacrilex): The
first nicotine prepa-ration that was made available for use is the nicotine gum
(available in the West as Nicorette 2
mg and 4 mg strengths). It is designed to be chewed slowly and intermittently.
Approximately 50 to 70% of the nicotine is absorbed through the buccal mucosa,
while additional amounts are absorbed through swallowed saliva. Peak plasma
concentration is reached 15 to 30 minutes after starting to chew the gum, as
compared with 1 to 2 minutes after initiating smoking. Chewing the gum too
rapidly and vigorously can raise nicotine concentrations to uncomfortable
levels producing adverse effects (especially if the patient is also smoking at
the same time). If the gum is inadvertently swallowed, there is no cause for undue
concern since the nicotine is released and absorbed slowly producing only low
blood concentrations. The actual efficacy of nicotine gum, and the dose and
duration of therapy are highly variable.
–– Nicotine transdermal patch: The
disadvantages of nicotine gum (frequent administrations, unsightly chewing, bad
taste, nausea, and dyspepsia) are mostly avoided by transdermal nicotine, which
is available as nicotine-releasing adhesive patches of varying sizes and
delivery rates. The nicotine patch is generally available in 3 sizes, 30 cm2,
20 cm2, and 10 cm2, which deliver 21 mg, 14 mg, and 7 mg of nicotine
respectively, over 16 or 24 hours. The nicotine is released either directly
through the skin or through a membrane system in contact with the skin. Side effects
are mild and include dose-related sleep disturbances, dyspepsias, myalgias, and
increased cough.
––
Nicotine spray: In 1996, a nicotine nasal spray was released in the
United States as an alternative to gum or patch. It is available as a metered
dose inhaler containing 100 mg of nicotine at 10 mg/ml, designed to deliver 200
equivalent puffs each releasing 0.5 mg of nicotine. Absorption occurs through
the nasal mucosa which may be affected to some extent in the presence of
rhinitis. The recommended dose is 2 sprays (one in each nostril) every ½ or 1
hour, subject to a maximum of 40 doses (80 puffs) in any 24-hour period. While
initial reports have been favourable, use of the spray is relatively unpleasant
and unsightly.
Subsequently, a nicotine metered-dose
oral inhaler was tested in the USA, designed to mimic smoking by providing
airway stimulation as well as nicotine replacement. Absorption occurs through
the buccal and pharyngeal mucosa, and respiratory tree (on slow deep
inhalation).
o Other therapies—
––
Clonidine: Clonidine is an alpha2-adrenergic agonist that has
been found useful in the treatment of alcohol and opiate withdrawal. Subsequent
studies on its utility in nicotine withdrawal have shown that it could be
effective in promoting abstinence from cigarettes also. Although the exact
mechanism is not clear, it is postulated that clonidine is effective for most
withdrawal syndromes because it inhibits noradrenergic neurons in the locus
ceruleus.* The usual dose recommended is 150 to 200 mcg/day for 1 month.
However, clonidine use is associated with a high incidence of adverse effects
including tachy-cardia, hypotension, headache, vertigo, sedation, and visual
disturbances. These are minimised by substituting oral therapy with transdermal
patches in much the same way as nicotine patches. There are recent reports of
very satisfactory results by combining both transdermal nicotine and clonidine,
since the former reduces behavioural withdrawal symptoms, while the latter
reduces craving.![]()
–– Antidepressants: Since it is well
known that smokers who stop smoking have a high incidence of depression,
antidepressants such as doxepin and sertraline have been tried with varying
degrees of success in combating nicotine withdrawal. The efficacy of these
drugs requires validation by further studies.
––
Nicotine agonists and antagonists: These drugs have the potential to
block the effect of nicotine, i.e. removing its reinforcing effect on smoking
behaviour. Lobeline, the first of these drugs to be studied in this regard, is
a partial agonist that binds weakly and competitively to nicotine receptor
sites. However, it has not been found to be very effective in practice, though
it continues to be sold abroad as a smoking cessation aid under the brand name CigArrest. Mecamylamine is a nicotine
receptorantagonist that is said to reduce the craving for ciga-rettes if
administered for more than 6 weeks, though it can produce unpleasant side
effects including abdominal cramps, constipation, and urinary retention.
Adverse effects can be minimised by combining mecamylamine with nicotine skin
patch.
Forensic Issues
·
Tobacco abuse has been described as being the most
widespread cause of death and disability worldwide than any single disease
entity. Apart from the health problems brought on by smoking and other forms of
tobacco use on the user himself, environmental tobacco smoke inhalation can
have delete-rious effects on other individuals as well. Environmental tobacco
smoke (ETS) consists of mainstream smoke, sidestream smoke, and vapour-phase
components that diffuse through cigarette paper into the environment. ETS
exposure occurs frequently in the home, in the workplace, and in other public
areas where smoking is allowed. The Government of India is making attempts at
prohibiting tobacco smoking in public, and a few states have begun to implement
this with varying success rates. Passive smoking can cause the following health
problems:
o Adults: Lung cancer, small airway
damage, worsening of angina, hypertension.
o Children: Bronchitis, pneumonia,
worsening of asthma, middle ear effusions, decreased height, sudden infant
death syndrome.
o Neonates: Prematurity, low birth
weight, neonatal death. The term “foetal tobacco syndrome” is applied in those
cases where the mother had smoked 5 or more cigarettes per day throughout the
pregnancy, had no evidence of hypertension during pregnancy, and the newborn
baby showed symmetrical growth retardation as manifested by low birth weight
(less than 2.5 kg), and a ponderal index* greater than 2.32.
·
As far as acute nicotine poisoning is concerned, the
circum-stances could be accidental, suicidal, or even homicidal.
o Accidental poisoning could occur in
children who play with old tobacco pipes, or who ingest cigarettes out of
curiosity. Severe poisoning can result from ingestion of just 2 or 3
cigarettes. Milder poisoning can result from “experimental smoking” by
adolescents. Accidental poisoning in horticulture due to the use of nicotine as
a pesticide was not uncommon in the past. Apart from occupational exposure to
nicotine spray, even fruits contaminated with nicotine used to reach the
general public. Careless storage of nicotine in containers which could be
mistaken for some other product also sometimes caused accidental poisoning.
o Similarly, suicidal ingestion of
nicotine pesticides used to be reported occasionally in the past, until such
preparations were withdrawn from use.
o Homicidal cases have always been
rare, though a few cases do find mention in medical literature.
·
Autopsy findings in death due to nicotine ingestion
includeAutopsy findings in death due to nicotine ingestion include
characteristic odour (of stale tobacco) in gastric contents, brownish froth at
the mouth and nose, congestion with brownish discolouration of oesophageal and
gastric mucosa, and intense congestion of liver and kidneys.
Related Topics