Chapter: Medical Surgical Nursing: Assessment and Management of Patients With Endocrine Disorders
Thyroid Function and Dysfunction - Management of Patients With Thyroid Disorders
THYROID
FUNCTION AND DYSFUNCTION
Various
hormones and chemicals are responsible for normal thy-roid function. Key among
them are thyroid hormone, calcitonin, and iodine.
Thyroid Hormone
The two separate hormones, thyroxine (T4) and triiodothyronine (T3), that are produced by the thyroid gland and that make up thy-roid hormone, are amino acids that have the unique property of containing
iodine molecules bound to the amino acid structure. T4 contains four iodine
atoms in each molecule, and T3 contains only three. These hormones are
synthesized and stored bound to proteins in the cells of the thyroid gland
until needed for release into the bloodstream. About 75% of bound thyroid
hormone is bound to thyroxine-binding globulin (TBG); the remaining bound thyroid
hormone is bound to thyroid-binding prealbumin and albumin.
ROLE OF IODINE
Iodine
is essential to the thyroid gland for synthesis of its hormones. In fact, the
major use of iodine in the body is by the thyroid, and the major derangement in
iodine deficiency is alteration of thyroid function. Iodide is ingested in the
diet and absorbed into the blood in the gastrointestinal tract. The thyroid
gland is extremely efficient in taking up iodide from the blood and
concentrating it within the cells, where iodide ions are converted to iodine
molecules, which react with tyrosine (an amino acid) to form the thyroid
hormones.
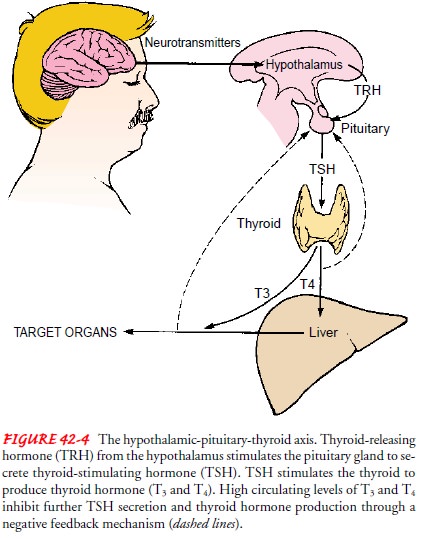
REGULATION OF THYROID HORMONE
The secretion of T3 and T4
by the thyroid gland is controlled by thyroid-stimulating hormone (TSH, or
thyrotropin) from the an-terior pituitary gland. TSH controls the rate of
thyroid hormone release. In turn, the level of thyroid hormone in the blood
determines the release of TSH. If thyroid hormone concentration in the blood
decreases, the release of TSH increases, which causes increased out-put of T3
and T4. This is an example of negative feedback.
Thyrotropin-releasing hormone (TRH), secreted
by the hypo-thalamus, exerts a modulating influence on the release of TSH from
the pituitary. Environmental factors, such as a decrease in temperature, may
lead to increased secretion of TRH, resulting in elevated secretion of thyroid
hormones. Figure 42-4 shows the hypothalamic-pituitary-thyroid axis, which
regulates thyroid hor-mone production.
FUNCTION OF THYROXINE AND TRIIODOTHYRONINE
The primary function of the thyroid hormone
is to control the cellular metabolic activity. T4, a relatively weak
hormone, main-tains body metabolism in a steady state. T3 is about
five times as potent as T4 and has a more rapid metabolic action.
These hor-mones accelerate metabolic processes by increasing the level of
specific enzymes that contribute to oxygen consumption and al-tering the
responsiveness of tissues to other hormones. The thy-roid hormones influence
cell replication and are important in brain development. Thyroid hormone is
also necessary for nor-mal growth. The thyroid hormones, through their
widespread ef-fects on cellular metabolism, influence every major organ system.
Calcitonin
Calcitonin, or thyrocalcitonin, is another important
hormone se-creted by the thyroid gland. It is secreted in response to high
plasma levels of calcium, and it reduces the plasma level of cal-cium by
increasing its deposition in bone.
Assessment and Diagnostic Findings
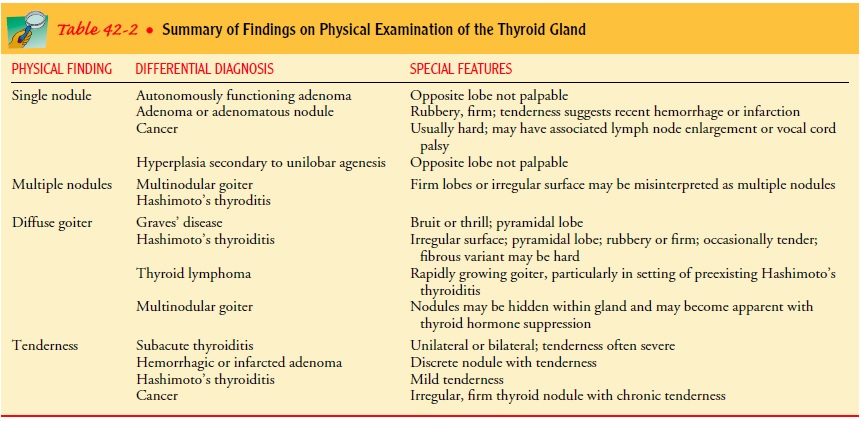
The thyroid gland is inspected and palpated routinely on all pa-tients. Inspection begins with identification of landmarks. The lower neck region between the sternocleidomastoid muscles is in-spected for swelling or asymmetry. The patient is instructed to extend the neck slightly and swallow. Thyroid tissue rises nor-mally with swallowing. The thyroid is then palpated for size, shape, consistency, symmetry, and the presence of tenderness.
The
examiner may examine the thyroid from an anterior or a posterior position. In
the posterior position, both hands encircle the patient’s neck. The thumbs rest
on the nape of the neck, while the index and middle fingers palpate for the
thyroid isthmus and the anterior surfaces of the lateral lobes. When palpable,
the isthmus is perceived as firm and of a rubber-band consistency.
The
left lobe is examined by positioning the patient so that the neck flexes
slightly forward and to the left. The thyroid cartilage is then displaced to
the left with the fingers of the right hand. This maneuver displaces the left
lobe deep into the sternocleidomastoid muscle, where it can be more easily
palpated. The left lobe is then palpated by placing the left thumb deep into
the posterior area of the sternocleidomastoid muscle, while the index and
middle fingers exert opposite pressure in the anterior portion of the muscle.
Hav-ing the patient swallow during the maneuver may assist the exam-iner to
locate the thyroid as it ascends in the neck. The procedure is reversed to
examine the right lobe. The isthmus is the only por-tion of the thyroid that is
normally palpable. If a patient has a very thin neck, two thin, smooth,
nontender lobes may also be palpable.
If
palpation discloses an enlarged thyroid gland, both lobes are auscultated using
the diaphragm of the stethoscope. Auscultation identifies the localized audible
vibration of a bruit. This abnor-mal finding indicates increased blood flow
through the thyroid gland and necessitates referral to a physician. Tenderness,
en-largement, and nodularity within the thyroid also require refer-ral for
additional evaluation (Table 42-2).
THYROID FUNCTION TESTS
Assessment
measures in addition to palpation and auscultation include thyroid function
tests, such as laboratory measurement of thyroid hormones, thyroid scanning,
biopsy, and ultrasonog-raphy. The most widely used tests are serum immunoassay
for TSH and free thyroxine (FT4). Measurement of TSH has a sen-sitivity and
specificity of greater than 95% (Larson, Anderson & Koslawy, 2000). FT4 levels correlate with metabolic status
and are elevated in hyperthyroidism and decreased in hypothyroidism.
THYROID-STIMULATING HORMONE
Measurement of the serum TSH concentration is
the single best screening test of thyroid function in outpatients because of
its high sensitivity. The ability to detect minute changes in serum TSH makes
it possible to distinguish subclinical thyroid disease from euthyroid states in
patients with low or high normal values. Val-ues above the normal range of 0.4
to 6.15 ÎĽU/mL
indicate pri-mary hypothyroidism, and low values indicate hyperthyroidism. When
the TSH is normal, there is a 98% chance that the FT4 is also
normal. Measurement of TSH is also used for monitoring thyroid hormone
replacement therapy and for differentiating be-tween disorders of the thyroid
gland itself and disorders of the pituitary or hypothalamus. Current
recommendations suggest TSH screening for all adults beginning at age 35, and
every 5 years thereafter (Ladenson, Singer, Ain, et al., 2000).
SERUM FREE THYROXINE
The test most commonly used to confirm an
abnormal TSH is FT4. FT4 is a direct measurement of free
(unbound) thyroxine, the only metabolically active fraction of T4.
The range of FT4 in serum is normally 0.9 to 1.7 ng/dL (11.5 to 21.8
pmol/L). When measured by the dialysis method, FT4 is not affected
by variations in protein binding and is the procedure of choice for following
the changes in T4 secretion during treatment of hyperthyroidism.
Measurement of FT4 by the immunoassay technique is less reli-able
because it may be affected by medication, illness, or changes
in protein binding. An estimate (or index) of
FT4 can also be cal-culated by multiplying total T4 by T3
resin uptake.
SERUM T3 AND T4
Measurement of total T3 or T4
includes protein-bound and free hormone levels that occur in response to TSH
secretion. T4 is 80% bound to thyroxine-binding globulin (TBG); T3
is bound less firmly. Only 0.03% of T4 and 0.3% of T3 is
unbound. Any factor that alters binding proteins also changes the T3
and T4 lev-els. Serious systemic illnesses, medications (eg, oral
contracep-tives, corticosteroids, phenytoin, salicylates), and protein wasting
as a result of nephrosis and use of androgens may interfere with accurate test
results. Normal range for T4 is 4.5 to 11.5 ÎĽg/dL (58.5 to 150 nmol/L). Although serum T3
and T4 levels generally increase or decrease together, the T3
level appears to be a more ac-curate indicator of hyperthyroidism, which causes
a greater rise in T3 than T4 levels. The normal range for
serum T3 is 70 to 220 ng/dL (1.15 to 3.10 nmol/L).
T3 RESIN UPTAKE TEST
The T3 resin uptake test is an
indirect measure of unsaturated TBG. Its purpose is to determine the amount of
thyroid hormone bound to TBG and the number of available binding sites. This
provides an index of the amount of thyroid hormone already pre-sent in the
circulation. Normally, TBG is not fully saturated with thyroid hormone, and
additional binding sites are available to combine with radioiodine-labeled T3
added to the blood speci-men. The normal T3 uptake value is 25% to
35% (relative uptake fraction, 0.25 to 0.35), which indicates that about one
third of the available sites of TBG are occupied by thyroid hormone. If the
number of free or unoccupied binding sites is low, as in hyperthyroidism, the T3
uptake is greater than 35% (0.35). If the number of available sites is high, as
occurs in hypothyroidism, the test results are less than 25% (0.25).
T3 uptake is useful in the evaluation of thyroid hormone levels in patients who have received diagnostic or therapeutic doses of iodine. The test results may be altered by the use of estrogens, an-drogens, salicylates, phenytoin, anticoagulants, or corticosteroids.
THYROID ANTIBODIES
Autoimmune
thyroid diseases include both hypothyroid and hyperthyroid conditions. Results
of testing by immunoassay tech-niques for antithyroid antibodies, specifically
antimicrosomal antibodies, are positive in chronic autoimmune thyroid disease
(90%), Hashimoto’s thyroiditis (100%), Graves’ disease (80%), and other
organ-specific autoimmune disease, such as lupus ery-thematosus and rheumatoid
arthritis. Antithyroid antibody titers are normally present in 5% to 10% of the
population and in-crease with age.
RADIOACTIVE IODINE UPTAKE
The radioactive iodine uptake test measures
the rate of iodine up-take by the thyroid gland. The patient is administered a
tracer dose of iodine-123 (123I) or another radionuclide, and a
count is made over the thyroid gland with use of a scintillation counter, which
detects and counts the gamma rays released from the breakdown of 123I
in the thyroid. It measures the proportion of the adminis-tered dose present in
the thyroid gland at a specific time after its administration. It is a simple
test and provides reliable results. It is affected by the patient’s intake of
iodide or thyroid hormone; therefore, a careful preliminary clinical history is
essential in eval-uating results. Normal values vary from one geographic region
to another and with the intake of iodine. Patients with hyper-thyroidism
exhibit a high uptake of the 123I (in some patients, up to 90%),
whereas patients with hypothyroidism exhibit a very low uptake. This test is
also used to determine what dose of 123I should be administered to
treat a patient with hyperthyroidism.
FINE-NEEDLE ASPIRATION BIOPSY
Using
a small-gauge needle to sample the thyroid tissue for biopsy is a safe and
accurate method of detecting malignancy. It is often the initial test for
evaluation of thyroid masses. Results are re-ported as (1) negative (benign),
(2) positive (malignant), (3) in-determinate (suspicious), and (4) inadequate
(nondiagnostic).
THYROID SCAN, RADIOSCAN, OR SCINTISCAN
In a thyroid scan, a scintillation detector
or gamma camera moves back and forth across the area to be studied in a series
of parallel tracks, and a visual image is made of the distribution of
radio-activity in the area being scanned. Although 123I has been the
most commonly used isotope, several other radioactive isotopes, including
technetium-99m (99mTc) pertechnetate, thallium, and americium, are
also used.
Scans are helpful in determining the
location, size, shape, and anatomic function of the thyroid gland, particularly
when thyroid tissue is substernal or large. Identifying areas of increased
function (“hot” areas) or decreased function (“cold” areas) can assist in
di-agnosis. Although most areas of decreased function do not repre-sent
malignancies, lack of function increases the likelihood of malignancy,
particularly if only one nonfunctioning area is present. Scanning of the entire
body, to obtain the total body profile, may be carried out in a search for a
functioning thyroid metastasis.
OTHER DIAGNOSTIC TESTS
Ultrasound, CT scans, and MRI may be used to
clarify or con-firm the results of other diagnostic studies. Thyroglobulin
(Tg), a precursor for T3 and T4, can be measured reliably
in the serum by radioimmunoassay. Clinically, it is used to detect persistence
or recurrence of thyroid carcinoma.
NURSING IMPLICATIONS
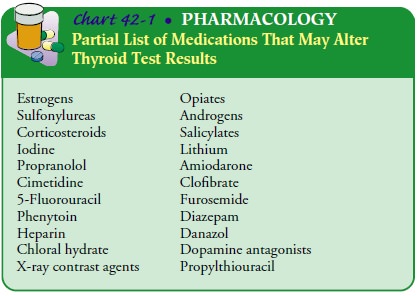
When
thyroid tests are scheduled, it is necessary to determine whether the patient
has taken medications or agents that contain iodine because these may alter the
test results. Iodine-containing medications include contrast agents and those
used to treat thyroid disorders. Less obvious sources of iodine are topical
antiseptics, multivitamin preparations, and food supplements frequently found
in health food stores; cough syrups; and amiodarone, an antiar-rhythmic agent.
Other medications that may affect test results are estrogens, salicylates,
amphetamines, chemotherapeutic agents, antibiotics, corticosteroids, and
mercurial diuretics. The nurse asks the patient about the use of these
medications and notes their use on the laboratory requisition. Chart 42-1 gives
a partial list of agents that may interfere with accurate testing of thyroid
gland function.
Related Topics