Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Communities, ecosystems, and the functional role of fishes
The effects of fishes on plants
The effects of fishes on plants
The activities of herbivores are usually divided into browsing versus grazing, which are different feeding modes that affect plants differently (Horn & Ferry-Graham 2006).Browsing involves removing parts of the plant on which the fish is feeding, such as the tips of leaves or the leaves themselves (e.g., Silver Dollar characins, many cichlids, damselfishes). Grazing involves biting the plant off at the substrate and even taking in some of the substrate itself, as in the case of parrotfishes that scrape coral surfaces in the process of eating algae. The ecological impacts of fishes on plants also vary among habitats, affecting plants by altering biomass, productivity, growth form, and species composition, by dispersing seeds, and by causing changes in the
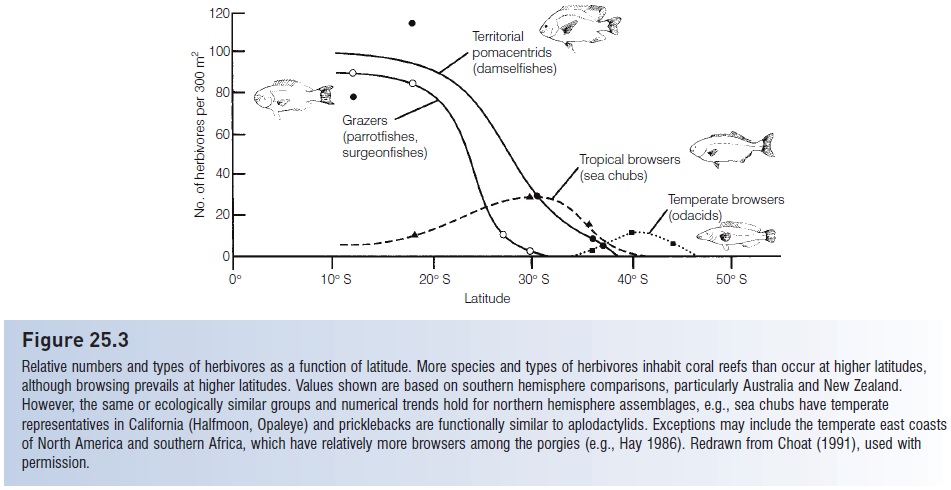
Figure 25.3
Relative numbers and types of herbivores as a function of latitude. More species and types of herbivores inhabit coral reefs than occur at higher latitudes, although browsing prevails at higher latitudes. Values shown are based on southern hemisphere comparisons, particularly Australia and New Zealand. However, the same or ecologically similar groups and numerical trends hold for northern hemisphere assemblages, e.g., sea chubs have temperate representatives in California (Halfmoon, Opaleye) and pricklebacks are functionally similar to aplodactylids. Exceptions may include the temperate east coasts of North America and southern Africa, which have relatively more browsers among the porgies (e.g., Hay 1986). Redrawn from Choat (1991), used with permission.
Herbivory is variably developed in different communities. Latitude appears to be the greatest determinant of herbivore diversity. Above 40° north or south, herbivores are rare or lacking in marine and freshwater fish assemblages (herbivory is variously defined by different authors, but generally it means that a fish’s diet consists of at least 25–50% plants). Herbivorous fishes are most diverse, are usually most dense, and make up a larger percentage of the assemblage in tropical than in temperate habitats. The number of species, relative abundance, and absolute density of herbivorous fishes often increases with decreasing latitude in a region (e.g., Floeter et al. 2004, 2005). Fewer than 25% of the species in a temperate stream are herbivorous, whereas 25–100% of the species in a tropical stream may be herbivorous. Temperate marine habitats contain 5–15% herbivorous species, whereas 30–50% of the species in coral reef assemblages are herbivorous (Horn 1989; Wootton & Oemke 1992) (Fig. 25.3). High-latitude herbivores exist, but their feeding behavior reflects the seasonal availability of plant material. At least one Antarctic icefish (Notothenia neglecta) eats macroalgae and diatoms during spring and summer, and switches to carnivory in fall and winter (Daniels 1982). Why herbivorous fishes, and fishes that rely on low-energy food sources such as detritus (e.g., mullets) are more common at lower latitudes remains a
matter of conjecture. Explanations include the seasonal nature of the food source, the energetics of extracting carbon from low-energy food sources at low temperatures, and the biogeography of herbivorous taxa (e.g., Harmelin- Vivien 2002; Floeter et al. 2005). Comparative studiesacross latitudinal gradients, such as those of Ferreira et al. (2004) and Floeter et al. (2004), need to be conducted in other regions and habitats.
Tropical communities
In tropical streams, the most common families containing herbivores are minnows, characins (particularly several piranha relatives), and cichlids, and to a lesser extent catfishes, livebearers, and gouramis (Goulding 1980; Lowe- McConnell 1987). In Panama, experimental manipulations have shown that algal biomass is reduced by the feeding activity of loricariid catfishes in both shallow (<20 cm) and deep habitats. When algal-covered rocks are transplanted from shallow regions where biomass is usually higher to deeper regions, the algae are quickly cropped by catfishes. When the catfishes are removed, algal growth is similar at both depths. Higher standing stocks of algae are maintained in shallows because predatory birds limit the feeding activity of the catfishes there (Power et al. 1989; see above). Work in Costa Rican streams with a fish assemblage of at least 13 species that eat plants suggests that fishes consume a significant fraction of the macrophytes (a general term for rooted aquatic plants), algae, and grasses, as well as the leaves that fall into the stream (Wootton & Oemke 1992; see below for a discussion of seed consumption and dispersal in tropical streams).
Continuous consumption of periphyton (the algal covering on rocks) and leaves may have a strong effect on the development of an aquatic insect fauna. Temperate streams typically have a much more diverse assemblage of aquatic insects than do tropical streams; many of these insects live in and feed on periphyton and leaves. The diversity and activities of herbivorous fishes in tropical streams may keep these resources at levels too low to permit the development of a diverse fauna of herbivorous aquatic insects, despite the incredible diversity of terrestrial insects in the tropics (e.g., Flecker & Allan 1984).
It is in tropical river systems that the role of fishes in dispersing seeds is best known. Fruiting of many trees coincides with the annual or semiannual flooding of the rivers (see Seasonal patterns). Hence fishes gain access to the base of the trees, or trees gain access to the water. In South and Central America, fruits and seeds that fall into the water are consumed by several characoid fishes, such as Pacu (Colossoma spp.) and Brycon guatemalensis, and constitute the major part of the diets of these fishes during those periods. Fruits and seeds of at least 40 different tree species are eaten by fishes in the Rio Machado region of the Amazon. Although some fruits and seeds may be killed by digestive processes, many seeds pass through the gut unharmed; germination may even be aided by the time spent in a fish’s gut. In the case of Brycon feeding on the seeds of a common riparian fi g tree, no loss in germination occurred. Importantly, seeds remained in the fish’s gut for 18–36 h, during which time some fish moved several kilometers. Hence consumption by fishes may aid dispersal of the tree’s seeds, including dispersal in the otherwise unobtainable upstream direction (Goulding 1980; Agami & Waisel 1988; Horn 1993).
Tropical lakes have a large number of herbivorous fishes, particularly among the minnows, characins, catfishes, and cichlids. Phytoplanktivorous fishes can affect the relative abundances of phytoplankton species, even though a large fraction of the ingested phytoplankton cells may pass through a fish’s gut unharmed (Miura & Wang 1985). Some cichlid species engage in suspension feeding, whereby they pump water into their mouth and out their gill openings, filtering out different size prey. In Blue Tilapia, Tilapia aurea, particles larger than 25 μm are retained by the gill rakers and by mucus-covered microbranchiospines on the gill arches; smaller particles pass through. In experimental ponds, large phytoplankton species are filtered out of the water and smaller species come to dominate and even increase in numbers. Small phytoplankters may thrive because of nutrient enrichment due to fish excretion and also because fish filter out zooplankton (rotifers, water fleas) that prey on the phytoplankton (see below).
Interactions between plants and fishes are probably best known on coral reefs, where herbivory has led to antiherbivore adaptations in plants and in turn to adaptations by fishes to overcome plant defenses (Choat & Clements 1998; see Fig. 25.3). Herbivore densities on reefs average 0.5 fish/m2, as compared to 0.1 fish/m2 in most temperate marine habitats. These values do not include the abundant sea urchins, snails, and microcrustaceans that also feed on reef plants (Horn 1989). Reef fishes affect plant biomass, productivity, species composition, distribution, and growth form; whether coral reef fishes distribute reproductive products is apparently unknown. Resident herbivorous fishes can consume most of the daily productivity of the algae on a reef and in the process stimulate higher production rates in cropped algae than in uncropped algal turfs (Carpenter 1986; Klumpp & Polunin 1989). Algae respond via adaptive changes in growth form. Algae subjected to cropping assume lower, spreading shapes, whereas an absence of herbivores leads to upright, foliose growth forms of the same species (e.g., Lithophyllum and Padina).
Defensive responses also commonly involve mechanical or chemical adjustments. Plants may be leathery or rubbery (e.g., Sargassum), or even hard, as in the case of coralline algae. Seaweeds also produce a variety of so-called “secondary compounds”, usually halogenated terpenoids, which are distasteful to fishes; more than 20 such compounds have been isolated from marine algae (e.g., caulerpenyne from Caulerpa, halimedatrial from Halimeda, and several dictyols from Dictyota). Freshwater fishes also avoid plants with abundant phenolic compounds (Lodge 1991). Some tropical plants respond both mechanically and chemically, such as the green alga Halimeda, that deposits calcium carbonate in its tissues and also produces distasteful chemicals. Some plant species always possess such defenses, whereas others produce secondary compounds in direct response to recent herbivore activity. Most algae with such noxious properties are generally avoided by herbivorous fishes, although some fish species appear to specialize on particularly tough or chemically defended plant species. Sea chubs prefer brown seaweeds such as Dictyota that contain dictyols and are generally avoided by most other reef fishes (Horn 1989; Hay 1991).
A possible antiherbivore adaptation may be the production of ciguatera toxins, a class of substances that was first noticed because of its effects on humans. When a person eats a ciguatoxic fish, adverse reactions include a variety of gastrointestinal, neurological, and cardiovascular symptoms, including reversal of sensations (e.g., ice cream feeling hot) and possibly death from respiratory failure. Over 400 species of reef fishes have been implicated in ciguatera poisoning, but the most lethal sources have been large predators such as moray eels, groupers, snappers, and barracuda. These circumstances suggested that the ciguatera toxin was magnified as it passed through the reef food chain. Extensive research has verified that the toxin or toxins originate in unicellular dinofl agellates, primarily in the genera Gambierdiscus, Ostreopsis, andProrocentrum (Landsberg 2002). These dinofl agellates grow as epiphytes on common reef macroalgae or on newly exposed coral surfaces, as occurs when reefs are disturbed during dredging, blasting, and ship anchoring. Herbivores ingest the dinoflagellates directly or indirectly when consuming plants. Herbivores such as parrotfishes and surgeonfishes can also be ciguatoxic. The toxin is apparently not broken down by
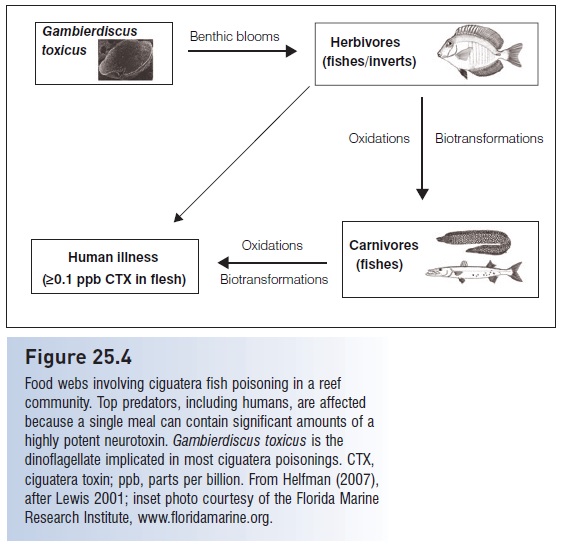
Figure 25.4
Food webs involving ciguatera fish poisoning in a reef community. Top predators, including humans, are affected because a single meal can contain significant amounts of a highly potent neurotoxin.Gambierdiscus toxicus is the dinoflagellate implicated in most ciguatera poisonings. CTX, ciguatera toxin; ppb, parts per billion. From Helfman (2007), after Lewis 2001;
Transmission of the toxin up the food chain may be facilitated because many fish species as well as crustaceans exhibit loss of equilibrium and erratic swimming after feeding on ciguatoxic plants and prey, which makes contaminated prey more susceptible to predation (Randall 1958; Davin et al. 1988; Kohler & Kohler 1994; Lewis2001). Drying, freezing, and cooking fail to denature the toxins, which allows exported fish to cause problems far from the tropics. Twenty people at a dinner in Calgary, Alberta, suffered ciguatera poisoning from eating thawedand- cooked reef fishes imported from Fiji (Haney 2002). No readily available, inexpensive means exist for testing for the toxin, although ToxiTecs, Inc. advertises the Cigua- Check® fish poison test kit . Clearly, the welfare of reef fish assemblages and reef communities can be directly linked to the integrity of the reef ecosystem itself. Where ciguatera is involved, human health is an added consideration.
One byproduct of differences in plant palatability, and a factor that can affect algal species composition on reefs, is the gardening behavior of damselfishes. The algal assemblage within a damselfish territory has been justly termed a “lawn” because it frequently consists of a few highly palatable algal species (e.g., the red alga,Polysiphonia) cropped down to a fairly even level; less desirable species are actively weeded out (Lassuy 1980; Irvine 1981). The lawn contrasts with the surrounding area, where abundant roving herbivores such as parrotfishes and surgeonfishes may graze most surfaces down to relatively bare rock or leave only crustose coralline algae. If heavy fishing pressure has removed large herbivores, surfaces outside damselfish territories may have a higher biomass of algae than inside such territories. Regardless, damselfishes, whose territories may cover 40– 50% of a reef ’s surface, and other territorial herbivores (parrotfishes, blennies, surgeonfishes) can have a substantial effect on overall algal species diversity and distribution (Hixon & Brostoff 1983; Klumpp et al. 1987; Horn 1989; Hay 1991).
Hixon and Brostoff (1983) found that damselfish territoriality led to higher levels of algal species diversity (Fig. 25.5). The feeding rates of damselfish inside their territories were less intense than the levels of herbivory in unguarded areas outside the territories where parrotfishes and surgeonfishes were abundant. Roving herbivores kept algal diversity low outside territories because they grazed many surfaces bare. In caged enclosures that excluded both damselfishes and other herbivores, algal diversity was again lower than in damselfish territories because certain algal species were able to overgrow and eliminate other species. Hence the damselfish serves as a “keystone” species whose activities increase algal diversity by decreasing the disturbance created by roving herbivores and also by decreasing a competitive dominant alga’s ability to monopolize the space resources of an area.
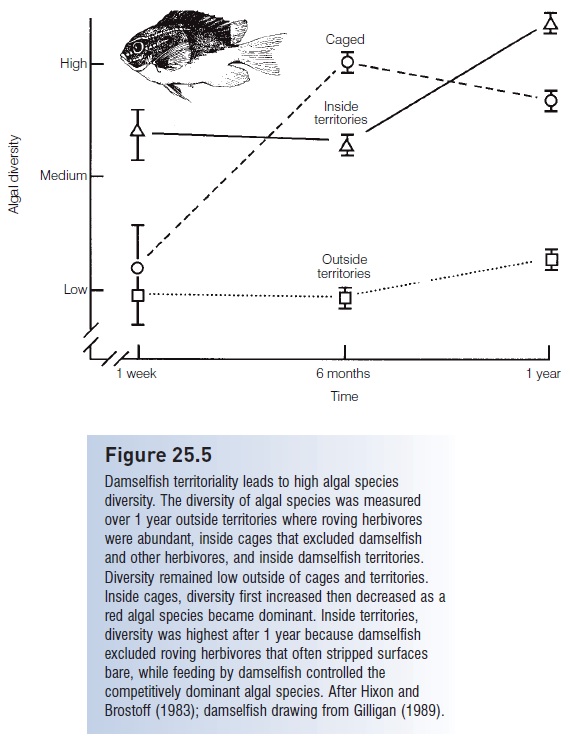
Figure 25.5
Damselfish territoriality leads to high algal species diversity. The diversity of algal species was measured over 1 year outside territories where roving herbivores were abundant, inside cages that excluded damselfish and other herbivores, and inside damselfish territories.
Diversity remained low outside of cages and territories. Inside cages, diversity first increased then decreased as a red algal species became dominant. Inside territories, diversity was highest after 1 year because damselfish excluded roving herbivores that often stripped surfaces bare, while feeding by damselfish controlled the competitively dominant algal species. After Hixon and Brostoff (1983); damselfish drawing from Gilligan (1989).
Temperate communities
In temperate streams and rivers, herbivory occurs primarily among minnows, catfishes, suckers, and killifishes. Perhaps 55 of the 950+freshwater fish species in North America can be classified as primarily herbivorous (Allan & Castillo 2007). Although only a few species are exclusively or even strongly herbivorous, some dominate locally in numbers or biomass and have a strong effect on algal growth (e.g., several pupfishes, Cyprinodon spp.; some suckers, Catostomus spp.; Brown Bullhead Catfish,Ameiurus nebulosus). The Stoneroller Minnow, Campostoma anomalum, can attain densities of 10 fish/m2 in midwestern US streams, each fish taking 10–15 bites/min at the algal substrate and consuming 27% of its mass in algae daily. This activity can reduce biomass, alter species composition, and increase growth rates of algal communities.
The thick overstory of filamentous green algae and diatoms is removed, leaving behind a thin layer of blue-green algae (cyanobacteria) that receives more light and more nutrients. Biomass-specific primary production is increased in areas grazed by minnows, which means that the plants left behind are more productive than those removed, probably because the algal types that are removed tend to be slow-growing forms and those left behind are faster-growing species (Matthews et al. 1987; Gelwick & Matthews 1992).
Few herbivores, especially at higher latitudes, are facultative plant consumers, even using the generous 25% plantmatter criterion to define the dietary guild (e.g., Allan & Castillo 2007). It is becoming increasingly evident that many if not most herbivorous fishes readily digest and probably actively seek out animal matter, either when taken in with plants or when opportunistically available. Even the weed-controlling Grass Carp (see below) requires some animal protein in its diet to achieve proper growth (Wiley & Wike 1986). Some omnivorous fishes consume large quantities of algae, particularly when preferred animal food is unavailable. Also, many carnivorous fishes can utilize algae as a supplemental food source when animal matter is unavailable (e.g., Kitchell & Windell 1970; Gunn et al. 1977). A Sonoran Desert minnow, the Longfin Dace (Agosia chrysogaster), prefers mayfl ies in the spring but takes in large quantities of algae in the fall when mayfl ies are no longer available. By increasing its feeding rate during the fall to allow for the lower nitrogen content of plant material, Longfin Dace maintain a relatively constant uptake rate of nitrogen. Nitrogen is then egested via feces and excreted across the gills into the stream in forms that are rapidly taken up by plants and may account for 10% of the nitrogen used by an otherwise nitrogen-limited stream ecosystem (Grimm 1988).
Herbivorous fishes in temperate lakes often belong to the same families as those that occur in rivers. Their impact on plant biomass can be considerable. Roach (Rutilus rutilus) and Rudd (Scardinius erythrophthalmus) in Lake Mikolajskie in Poland consume 1700 kg of macrophytes and 3800 kg of filamentous algae per hectare per year; the macrophyte values amount to about 15% of the annual total biomass (Prejs 1984). Strong selectivity is shown; Roach and Rudd prefer Elodea over Potamogeton, consuming 34% of the former and only 0.1% of the biomass of the latter, despite the low relative abundance of Elodea. An optimality analysis of benefits (amount of plant obtainable, nutritive value) and costs (plant toughness, availability of leaves and plants) indicates that the preference for Elodea exists because it provides a very high benefit : cost ratio as compared to other plant species.
The relative effectiveness of herbivorous lake fishes in consuming plant biomass is attested to by the widespread popularity and success of introductions of the Grass Carp,Ctenopharyngodon idella. Grass Carp, a native of China, have been widely introduced in the USA, Europe, and elsewhere to control unwanted aquatic plants, many of which are also introductions (e.g., Eurasian watermilfoil, Hydrilla, water hyacinth). Whereas most herbivorous fishes browse only the leaves of a plant and may in fact stimulate later growth, Grass Carp uproot and eat the entire plant. Grass Carp grow to a large size (to 30 kg) and consume 70–80% of their body weight daily; they can consequently eliminate all macrophytes in a lake, as happened in a Texas lake where 3650 ha of vegetation were eradicated within 2 years (Martyn et al. 1986). Total elimination of macrophytes is usually undesirable because, among other effects, it results in the destruction of critical habitat for invertebrates, amphibians, and juvenile fishes (Allen & Wattendorf 1987; Murphy et al. 2002). Feeding preferences by Grass Carp can also lead to shifts in the relative abundances of different species, altering species compositions within the plant assemblage. In experimental ponds, Grass Carp reduced total plant biomass by feeding preferentially on Chara, Elodea, andPotamogeton pectinatus. Later, total plant biomass increased over original conditions because those species avoided by the Grass Carp (Myriophyllum and P. natans) occupied the space vacated by the preferred plants. When Grass Carp consume submerged vegetation, floating leafed plants can come to dominate (Fowler & Robson 1978; Shireman et al. 1986).
Phytoplanktivory also occurs in temperate lakes and can lead to reduction in phytoplankton abundance. Gizzard Shad, Dorosoma cepedianum, selectively remove larger phytoplankton species (Drenner et al. 1984a, 1984b). One form of phytoplanktivory, suspension feeding (see above), has been documented in numerous temperate as well as tropical families, including many commercially important species (e.g., herrings, anchovies, whitefishes, minnows, silversides, mullets, cichlids, mackerels; Lazzaro 1987). Particles can be captured on structures other than the gill rakers. A cyprinid, the Blackfish (Orthodon microlepidotus), captures particles on its palatal organ, a mucus-covered region in the roof of the mouth (Sanderson et al. 1991).
Herbivores in temperate marine habitats are often abundant, although species diversity is lower than on tropical reefs (Horn & Ojeda 1999; Horn & Ferry-Graham 2006; see Fig. 25.3). Many porgies are seasonal herbivores that take advantage of plant growth during warmer months. Temperate porgies can become particularly abundant, achieving densities of more than 7 fish/m2 (Hay 1986). Temperate herbivores are usually browsers, eating the ends of algal fronds and other parts of seaweeds. Despite high seasonal abundance, available evidence indicates that temperate herbivores do not exercise the strong influence on algal ecology that is so evident in tropical marine environments. The strongest effects may be on the establishment and growth of young plants, as in the case of three California sea chub species that feed on young giant kelp, Macrocystis.
Territorial herbivores are generally lacking in temperate habitats, perhaps because a seasonally limited and variable plant resource makes territoriality impractical for most of the year (Horn 1989). Herbivory in temperate marine and fresh waters may be important, but invertebrates again probably exercise a greater influence than fishes. The association of “kelpbed” fishes with kelp beds is probably more of an attraction to the physical structure, refuge, and invertebrate production of the kelp than a dependence on the algae itself, with certain species and life history stages showing a stronger dependence on kelp than others (e.g., Kelp Perch, Brachyistius frenatus; Giant Kelpfish,Heterostichus rostratus; Kelp Clingfish, Rimicola muscarum; Kelp Rockfish, Sebastes atrovirens; Stephens et al. 2006); among
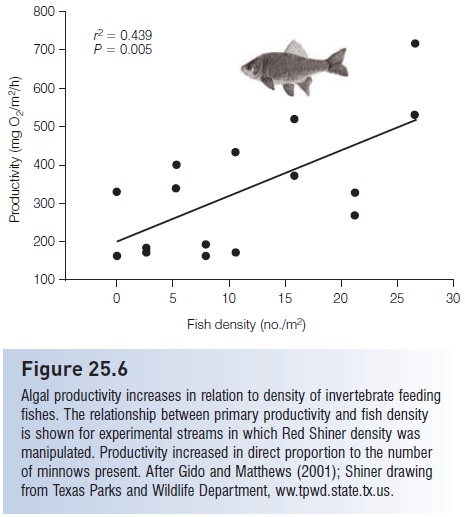
Figure 25.6
Algal productivity increases in relation to density of invertebrate feeding fishes. The relationship between primary productivity and fish density is shown for experimental streams in which Red Shiner density was manipulated. Productivity increased in direct proportion to the number of minnows present. After Gido and Matthews (2001); Shiner drawing from Texas Parks and Wildlife Department, ww.tpwd.state.tx.us.
The impact of fishes on plants may be an indirect one mediated by nutrient mobilization. For example, Red Shiners, Cyprinella lutrensis, in midwestern US streams feed heavily on insects that fall into the water. When shiner density was manipulated in experimental streams, benthic primary production increased in response to fish density, increasing 2.5-fold at high but natural densities of 27 fish/m2 (Gido & Matthews 2001) (Fig. 25.6). Hence, water column minnows that feed on drifting insects can affect primary productivity by transferring nutrients from terrestrial sources to benthic producers in stream ecosystems (see also below, Nutrient cycling and transport by fishes).
Related Topics