Chapter: Biochemistry: Electron Transport and Oxidative Phosphorylation
The Role of Electron Transport in Metabolism
The Role of Electron Transport in
Metabolism
Aerobic metabolism is a highly efficient way for an organism to
extract energy from nutrients. In eukaryotic cells, the aerobic processes
(including conversion of pyruvate to acetyl-CoA, the citric acid cycle, and
electron transport) all occur in the mitochondria, while the anaerobic process,
glycolysis, takes place outside the mitochondria in the cytosol. The reactions
of the electron transport chain take place in the inner mitochondrial membrane.
What is the importance of mitochondrial structure in ATP production?
The energy released by the oxidation of nutrients is used by
organisms in the form of the chemical energy of ATP. Production of ATP in the mitochondria
give ATP. The production of ATP by oxidative phosphorylation (an endergonic
process) is separate from electron transport to oxygen (an exergonic process),
but the reactions of the electron transport chain are strongly linked to one
another and are tightly coupled to the synthesis of ATP by phosphorylation of
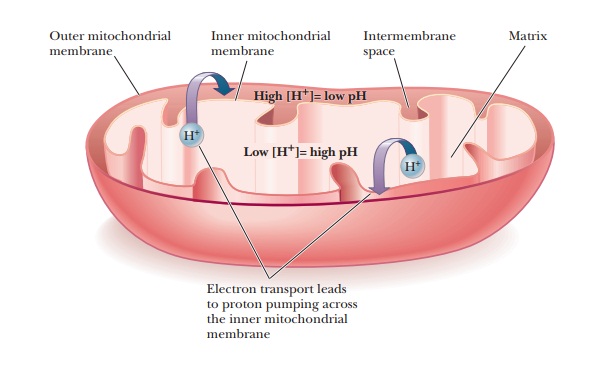
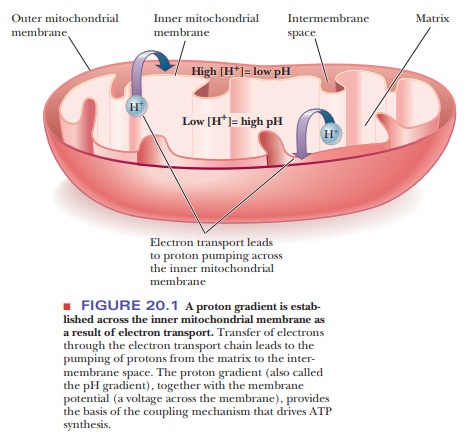
ADP. The operation of the electron transport chain leads to pumping of protons
(hydrogen ions) across the inner mitochondrial membrane, creating a pH gradient
(also called a proton gradient).
This proton gradient represents stored potential energy and provides the basis
of the coupling mechanism (Figure 20.1). Chemiosmotic
coupling is the name given to this mechanism. Oxidative phosphorylation
gives rise to most of the ATP production associated with the complete oxidation
of glucose.
The NADH and FADH2 molecules generated in
glycolysis and the citric acid cycle transfer electrons to oxygen in the series
of reactions known collectively as the electron transport chain. The NADH and FADH are oxidized to NAD+ and FAD
and can be used again in various metabolic pathways. Oxygen, the ultimate
electron acceptor, is reduced to water; this completes the process by which
glucose is completely oxidized to carbon dioxide and water.
We have already seen how carbon dioxide is produced from pyruvate,
which in turn is produced from glucose by the pyruvate dehydrogenase complex
and the citric acid cycle.
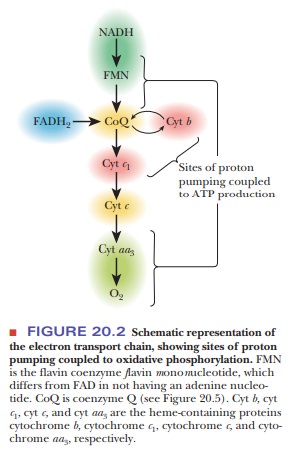
The complete series of oxidation–reduction reactions of the electron trans- port chain is presented in schematic form in Figure 20.2. A particularly note- worthy point about electron transport is that, on average, 2.5 moles of ATP are generated for each mole of NADH that enters the electron transport chain, and, on average, 1.5 moles of ATP are produced for each mole of FADH2.
The general outline of the process is that NADH passes electrons to
coenzyme Q, as does FADH2, providing an alternative
mode of entry into the electron trans-port chain. Electrons are then passed
from coenzyme Q to a series of proteins called cytochromes (which are
designated by lowercase letters) and, eventually, to oxygen.
Summary
Electron transport from one carrier to another creates a proton
gradient across the inner mitochondrial membrane.
The proton gradient is coupled to the production of ATP in aerobic metabolism
Related Topics