Chapter: Biochemistry: Electron Transport and Oxidative Phosphorylation
Organization of Electron Transport Complexes
Organization of Electron
Transport Complexes
Intact mitochondria isolated from cells can carry out all the
reactions of the electron transport chain; the electron transport apparatus can
also be resolved into its component parts by a process called fractionation.
Four separate respiratory complexes can
be isolated from the inner mitochondrial membrane.These complexes are
multienzyme systems. We encountered other examples of such multienzyme
complexes, such as the pyruvate dehydrogenase complex and the α-ketoglutarate dehydrogenase
complex. Each of the respiratory complexes can carry out the reactions of a
portion of the electron transport chain.
What reactions take place in the respiratory complexes?
Complex I The first complex,NADH-CoQ oxidoreductase,catalyzes the
firststeps of electron transport, namely the transfer of electrons from NADH to
coenzyme Q (CoQ). This complex is an
integral part of the inner mitochondrialmembrane and includes, among other
subunits, several proteins that contain an iron–sulfur cluster and the
flavoprotein that oxidizes NADH. (The total number of subunits is more than 20.
This complex is a subject of active research, which has proven to be a
challenging task because of its complexity. It is particularly difficult to
generalize about the nature of the iron–sulfur clusters because they vary from
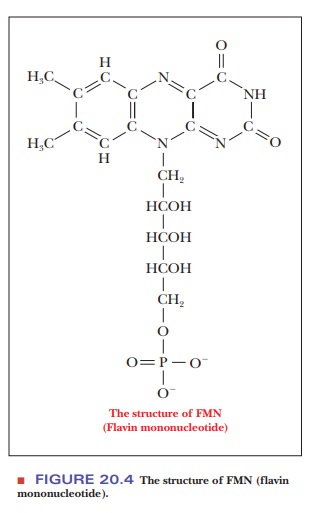
species to species.) The flavoprotein has a flavin coenzyme, called flavin
mononucleotide, or FMN, which differs from FAD in not having an adenine
nucleotide (Figure 20.4).
The
reaction occurs in several steps, with successive oxidation and reduction of
the flavoprotein and the iron–sulfur moiety. The first step is the transfer of
electrons from NADH to the flavin portion of the flavoprotein:
NADH + H+
+ E—FMN - > NAD+ + E—FMNH2
in which
the notation E—FMN indicates that the flavin is covalently bonded to the
enzyme. In the second step, the reduced flavoprotein is reoxidized, and the
oxidized form of the iron–sulfur protein is reduced. The reduced iron–sulfur
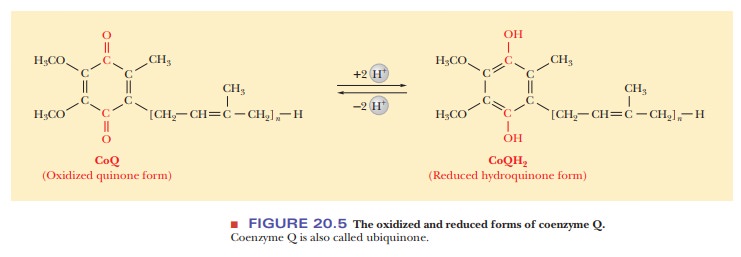
protein then donates its electrons to coenzyme Q, which becomes reduced to CoQH2
(Figure 20.5). Coenzyme Q is also called ubiquinone. The equations for the
second and third steps are shown here:
E—FMNH2
+ 2Fe—Soxidized - > E—FMN + 2Fe—Sreduced + 2H+
2Fe—Sreduced
+ CoQ + 2H+ - > 2Fe—Soxidized + CoQH2
The
notation Fe—S indicates the iron–sulfur clusters. The overall equation for the
reaction is
NADH + H+
+ CoQ - > NAD+ + CoQH2
This
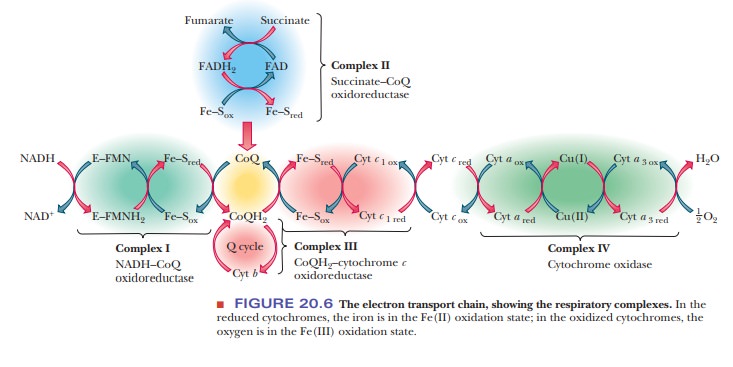
reaction is one of the three responsible for the proton pumping (Figure 20.6)
that creates the pH (proton) gradient. The standard free-energy change ( ∆G°' = –81 kJ mol–1 = –19.4
kcal mol–1) indicates that the reaction is strongly exergonic,
releasing enough energy to drive the phosphorylation of ADP to ATP
An important consideration about proton pumping and electron transport
is the subtle differences between the electron carriers. Although they can all
exist in an oxidized or reduced form, they reduce each other in a certain
order. In other words, reduced NADH donates its electrons to coenzyme Q, but
not the other way around. Thus, there is a direction to the electron flow in
the complexes we will study.
The
other important subtlety is that some carriers, such as NADH, carry electrons
and hydrogens in their reduced forms; others, such as the iron– sulfur protein
we just saw, can carry only electrons. This is the basis of the pro-ton pumping
that ultimately leads to ATP production. When a carrier such as NADH reduces
the iron–sulfur protein, it passes along its electrons, but not its hydrogens.
The architecture of the inner mitochondrial membrane and the electron carriers
allows the hydrogen ions to pass out on the opposite side of the membrane.
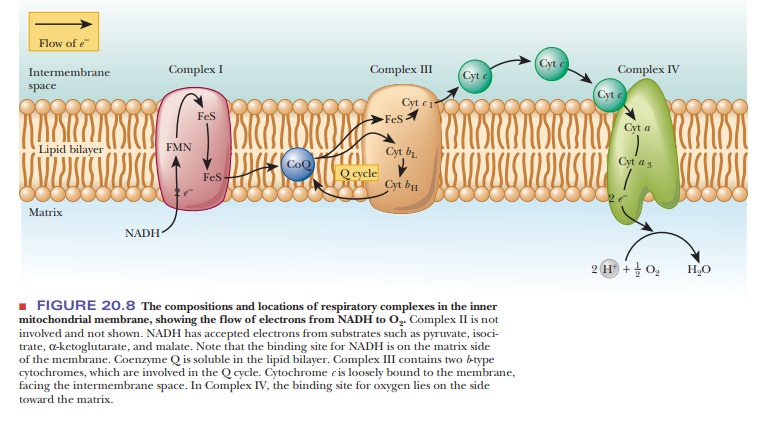
The final electron receptor of complex I, coenzyme Q, is mobile—that is to say, it is free to move in the membrane and to pass the electrons it has gained to the third complex for further transport to oxygen. We shall now see that the second complex also transfers electrons from an oxidizable substrate to coenzyme Q.
Complex II The second of the four
membrane-bound complexes,succinate-CoQ
oxidoreductase, also catalyzes the transfer of electrons to coenzyme
Q.However, its source of electrons (in other words, the substance being
oxidized) differs from the oxidizable substrate (NADH) acted on by NADH-CoQ
oxidoreductase. In this case the substrate is succinate from the citric acid
cycle, which is oxidized to fumarate by a flavin enzyme (see Figure 20.6).
Succinate
+ E—FAD - > Fumarate + E—FADH2
The
notation E—FAD indicates that the flavin portion is covalently bonded to the
enzyme. The flavin group is reoxidized in the next stage of the reaction as
another iron–sulfur protein is reduced:
E—FADH2
+ Fe—Soxidized - > E—FAD + Fe—Sreduced
This
reduced iron–sulfur protein then donates its electrons to oxidized coenzyme Q,
and coenzyme Q is reduced.
Fe—Sreduced
+ CoQ + 2H+ - > Fe—Soxidized + CoQH2
The
overall reaction is
Succinate
+ CoQ - > Fumarate + CoQH2
We
already saw the first step of this reaction when we discussed the oxidation of
succinate to fumarate as part of the citric acid cycle. The enzyme
traditionally called succinate dehydrogenase, which catalyzes the oxidation of
succinate to fumarate, has been shown by later work to be a part of this enzyme
complex. Recall that the succinate dehydrogenase portion consists of a
flavoprotein and an iron–sulfur protein. The other components of Complex
are a β-type
cytochrome and two iron–sulfur proteins. The whole complex is an integral part
of the inner mitochondrial membrane. The standard free-energy change ( ∆G°') is –13.5 kJ mol–1 =
–3.2 kcal mol–1. The overall reaction is exergonic, but there is not
enough energy from this reaction to drive ATP production, and no hydrogen ions
are pumped out of the matrix during this step.
In
further steps of the electron transport chain, electrons are passed from
coenzyme Q, which is then reoxidized, to the first of a series of very similar
pro-teins called cytochromes. Each
of these proteins contains a heme group, and in each heme group the iron is
successively reduced to Fe(II) and reoxidized to Fe(III). This situation
differs from that of the iron in the heme group of hemoglobin, which remains in
the reduced form as Fe(II) through the entire process of oxygen transport in
the bloodstream. There are also some structural differences between the heme
group in hemoglobin and the heme groups in the various types of cytochromes.
The
successive oxidation–reduction reactions of the cytochromes
Fe(III) + e – 3 Fe(II) (reduction)
and
Fe(II) 3 Fe(III) + e – (oxidation)
differ
from one another because the free energy of each reaction, ∆G°′, differs from the others because of the
influences of the various types of hemes and protein structures. Each of the
proteins is slightly different in structure, and thus each protein has slightly
different properties, including the tendency to participate in
oxidation–reduction reactions. The different types of cytochromes are
distinguished by lowercase letters (a, b,
c); further distinctions are possible with subscripts, as in c1.
Complex III The third complex, CoQH2- cytochrome c oxidoreductase (alsocalled
cytochrome reductase), catalyzes the oxidation of reduced coenzyme Q (CoQH2).
The electrons produced by this oxidation reaction are passed along to
cytochrome c in a multistep process.
The overall reaction is
CoQH2
+ 2 Cyt c[Fe(III)] - > CoQ + 2 Cyt
c[Fe(II)] + 2H+
Recall
that the oxidation of coenzyme Q involves two electrons, whereas the reduction
of Fe(III) to Fe(II) requires only one electron. Therefore, two molecules of
cytochrome c are required for every
molecule of coenzyme Q. The components of this complex include cytochrome b (actually two β-type cytochromes,
cytochrome bH and bL), cytochrome c1, and several iron–sulfur
proteins (Figure 20.6). Cytochromes can carry electrons, but not hydrogens.
This is another location where hydrogen ions leave the matrix. When reduced
CoQH2 is oxidized to CoQ, the hydrogen ions pass out on the other
side of the membrane.
The
third complex is an integral part of the inner mitochondrial mem-brane.
Coenzyme Q is soluble in the lipid component of the mitochondrial membrane. It
is separated from the complex in the fractionation process that resolves the
electron transport apparatus into its component parts, but the coenzyme is
probably close to respiratory complexes in the intact membrane (Figure 20.8). Cytochrome
c itself is not part of the complex
but is loosely bound to the outer surface of the inner mitochondrial membrane,
facing the intermembrane space. It is noteworthy that these two important
electron carri-ers, coenzyme Q and cytochrome c, are not part of the respiratory complexes but can move freely in
the membrane. The respiratory complexes themselves move within the membrane,
and electron transport occurs when one complex encounters the next complex in
the respiratory chain as they move.
The flow
of electrons from reduced coenzyme Q to the other components of the complex
does not take a simple, direct path. It is becoming clear that a cyclic flow of
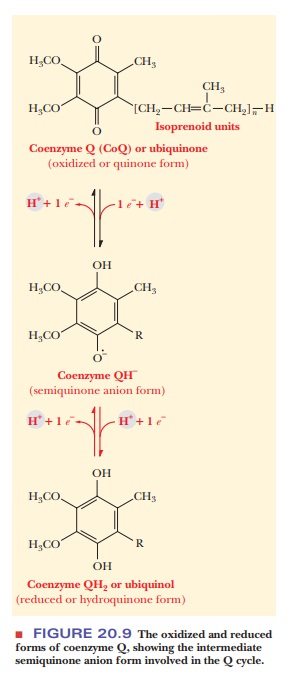
electrons involves coenzyme Q twice. This behavior depends on the fact that, as
a quinone, coenzyme Q can exist in three forms (Figure 20.9). The semiquinone
form, which is intermediate between the oxidized and reduced forms, is of
crucial importance here. Because of the crucial involve-ment of coenzyme Q,
this portion of the pathway is called the Q
cycle.
In part
of the Q cycle, one electron is
passed from reduced coenzyme Q to the iron–sulfur clusters to cytochrome c1, leaving coenzyme Q in the
semiqui-none form.
CoQH2
- > Fe—S - > Cyt c1
The
notation Fe—S indicates the iron–sulfur clusters. The series of reactions
involving coenzyme Q and cytochrome c1,
but omitting the iron–sulfur proteins, can be written as
CoQH2 + Cyt c1(oxidized) - > Cyt c1(reduced) + CoQ– (semiquinone anion) + 2H+
The
semiquinone, along with the oxidized and reduced forms of coenzyme Q,
participates in a cyclic process in which the two b cytochromes are reduced and oxidized in turn. A second molecule
of coenzyme Q is involved, transferring a second electron to cytochrome c1, and from there to the
mobile carrier cytochrome c. We are
going to omit a number of details of the process in the interest of simplicity.
Each of the two molecules of coenzyme Q involved in the Q cycle loses one
electron. The net result is the same as if one molecule of CoQ had lost two
electrons. It is known that one molecule of CoQH2 is regenerated,
and one is oxidized to CoQ, which is consistent with this picture. Most
important, the Q cycle provides a mechanism for electrons to be transferred one
at a time from coenzyme Q to cytochrome c1.
Proton
pumping, to which ATP production is coupled, occurs as a result of the
reactions of this complex. The Q cycle is implicated in the process, and the
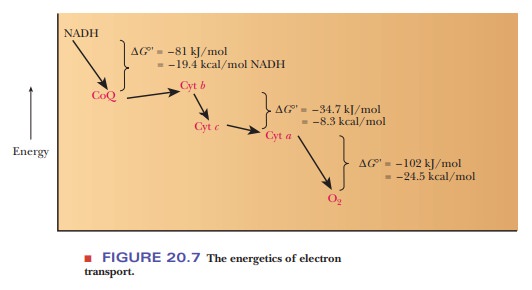
whole topic is under active investigation. The standard free-energy change ( ∆G°′) is –34.2 kJ = –8.2 kcal for each mole of NADH that enters the
electron transport chain (see Figure 20.7). The phosphorylation of ADP requires
30.5 kJ mol−1 = 7.3 kcal mol−1, and the reaction catalyzed by the third
complex supplies enough energy to drive the production of ATP.
Complex IV The fourth complex, cytochrome c oxidase,catalyzes the finalsteps of electron transport, the
transfer of electrons from cytochrome c
to oxygen.
The
overall reaction is
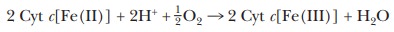
Proton
pumping also takes place as a result of this reaction. Like the other
respiratory complexes, cytochrome oxidase is an integral part of the inner
mitochondrial membrane and contains cytochromes a anda3, as
well as two Cu2+ ions that are involved in the electron transport
process. Taken as a whole, this complex contains about 10 subunits. In the flow
of electrons, the copper ions are intermediate electron acceptors that lie
between the two α-type cytochromes in the sequence
Cyt c - > Cyt a - > Cu2+ - > Cyt a3 - > O2
To show
the reactions of the cytochromes more explicitly,
Cyt c [reduced, Fe(II)] + Cyt aa3 [oxidized, Fe(III)] - > Cyt aa3 [reduced, Fe(II)] + Cyt c [oxidized, Fe(III)]
Cytochromes
a anda3 taken together form the complex known as cytochrome
oxidase. The reduced cytochrome oxidase is then oxidized by oxygen, which is
itself reduced to water. The half reaction for the reduction of oxygen (oxygen
acts as an oxidizing agent) is
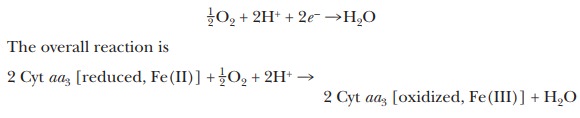
Note that in this final reaction we have
finally seen the link to molecular oxygen in aerobic metabolism.
The
standard free-energy change ( ∆G°')
is –110 kJ = –26.3 kcal for each mole of NADH that enters the electron transport
chain (see Figure 20.7). We have now seen the three places in the respiratory
chain where electron transport is coupled to ATP production by proton pumping.
These three places are the NADH dehydrogenase reaction, the oxidation of
cytochrome b, and the reac-tion of
cytochrome oxidase with oxygen, although the mechanism for proton transfer in
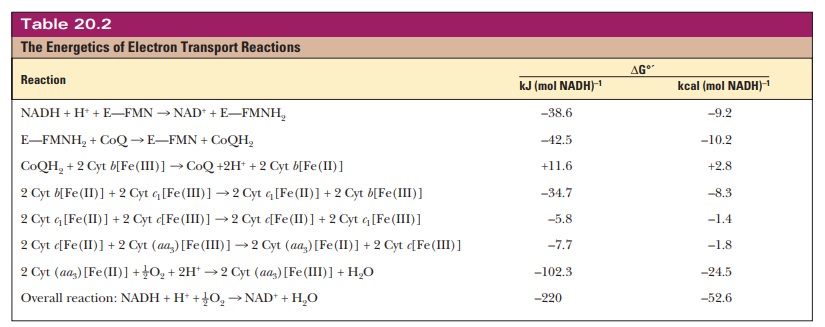
cytochrome oxidase remains a mystery. Table 20.2 summarizes the energetics of
electron transport reactions.
What is the nature of the iron-containing proteins of electron transport?
In
contrast to the electron carriers in the early stages of electron transport,
such as NADH, FMN, and CoQ, the cytochromes are macromolecules. These proteins
are found in all types of organisms and are typically located in membranes. In
eukaryotes, the usual site is the inner mitochondrial membrane, but cytochromes
can also occur in the endoplasmic reticulum.
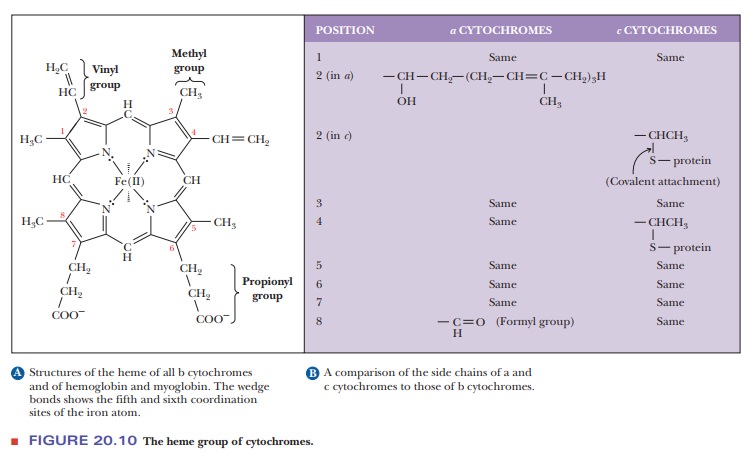
All
cytochromes contain the heme group, which is also a part of the struc-ture of
hemoglobin and myoglobin. In the cytochromes, the iron of the heme group does
not bind to oxygen; instead, the iron is involved in the series of redox
reactions, which we have already seen. There are differences in the side chains
of the heme group of the cytochromes involved in the various stages of electron
transport (Figure 20.10). These structural differences, com-bined with the
variations in the polypeptide chain and in the way the polypep-tide chain is
attached to the heme, account for the differences in properties among the
cytochromes in the electron transport chain.
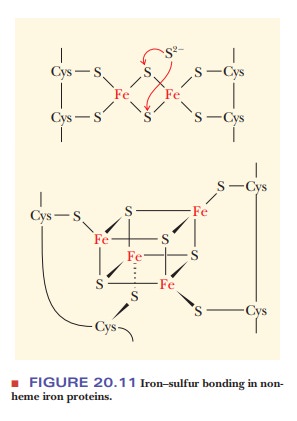
Nonheme iron proteins do not contain a heme group, as their name
indi-cates. Many of the most important proteins in this category contain
sulfur, as is the case with the iron–sulfur proteins that are components of the
respiratory complexes. The iron is usually bound to cysteine or to S2–
(Figure 20.11). There are still many questions about the location and mode of
action of iron–sulfur proteins in mitochondria.
Summary
The electron transport chain consists of four multisubunit
membrane-bound complexes and two mobile electron carriers (coenzyme Q and
cytochrome c). The reactions that
take place in three of these complexes generate enough energy to drive the
phosphorylation of ADP to ATP.
Many proteins of the electron transport chain contain iron, either
as part of a heme or combined with sulfur.
Related Topics