Chapter: Biochemistry: Electron Transport and Oxidative Phosphorylation
Respiratory Inhibitors Can Be Usedto Study Electron Transport
Respiratory Inhibitors Can Be
Usedto Study Electron Transport
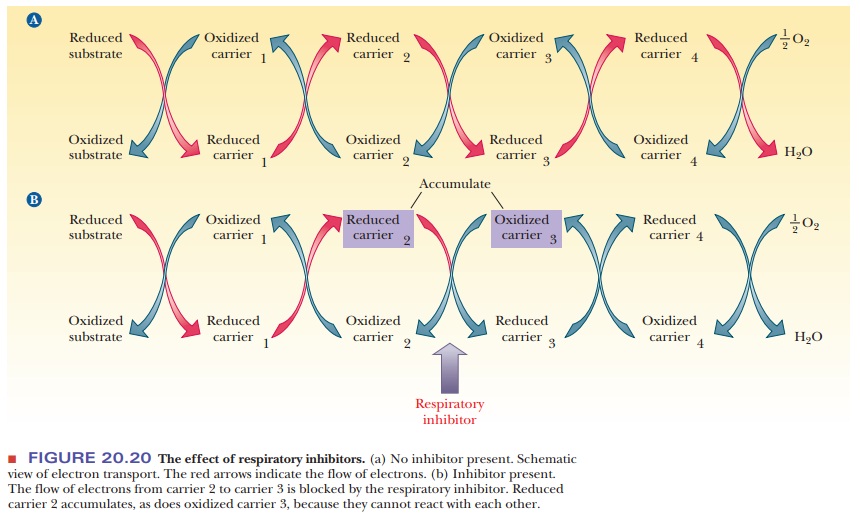
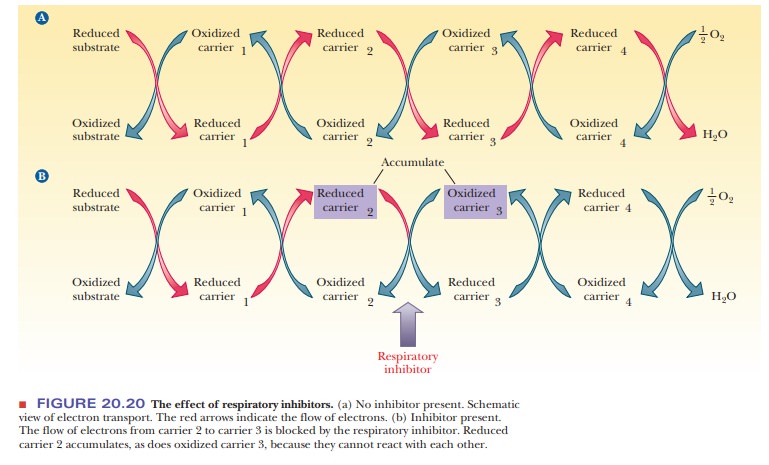
If a pipeline is blocked, a backup occurs. Liquid accumulates
upstream of the blockage point, and there is less liquid downstream. In
electron transport, the flow of electrons is from one compound to another
rather than along a pipe, but the analogy of a blocked pipeline can be useful
for understanding the workings of the pathway. When a flow of electrons is
blocked in a series of redox reactions, reduced compounds accumulate before the
blockage point in the pathway. Recall that reduction is a gain of electrons,
and oxidation represents a loss of electrons. The compounds that come after the
blockage point will lack electrons and will tend to be found in the oxidized
form (Figure 20.20). By using respiratory
inhibitors, we can gather additional evidence to establish the order of
components in the electron transport pathway.
Do respiratory inhibitors have a connection with respiratory complexes?
The use of respiratory inhibitors to determine the order of the
electron transport chain depends on determining the relative amounts of
oxidized and reduced forms of the various electron carriers in intact
mitochondria. The logic of the experiment can be seen from the analogy of the
blocked pipe. In this case, the reduced form of the carrier upstream (reduced carrier
2) accumulates because it cannot pass electrons farther in the chain. Likewise,
the oxidized form of the carrier downstream (oxidized carrier 3) also
accumulates because the supply of electrons that it could accept has been cut
off (Figure 20.20). By use of careful techniques, intact mitochondria can be
isolated from cells and can carry out electron transport if an oxidizable
substrate is available. If electron transport in mitochondria occurs in the
presence and absence of a respiratory inhibitor, different relative amounts of
oxidized and reduced forms of the electron carriers will be present.
The type of experiment done to determine the relative amounts of oxidized and reduced forms of electron carriers depends on the spectroscopic properties of these substances.
The oxidized and
reduced forms of cytochromes can be distinguished from one another. Specialized
spectroscopic techniques exist to detect the presence of electron carriers in intact mitochondria. The
indi-vidual types of cytochromes can be identified by the wavelength at which
the peak appears, and the relative amounts can be determined from the
intensities of the peaks.
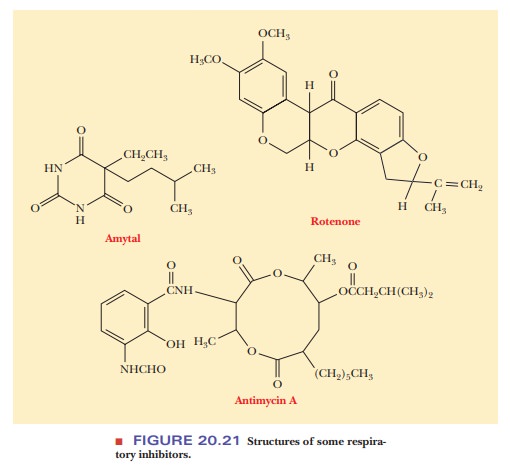
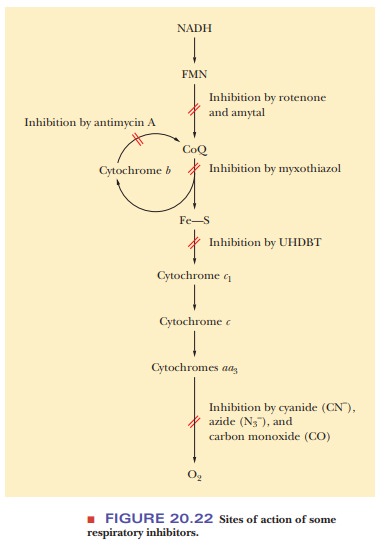
Inhibitors
have an effect at three sites in the electron transport chain, and we shall
look at some classic examples. At the first site, barbiturates (of which amytal
is an example) block the transfer of electrons from the flavoprotein NADH
reductase to coenzyme Q. Rotenone is another inhibitor that is active at this site.
This compound is used as an insecticide; it is highly toxic to fish, but not to
humans, and is often used to kill the fish in a lake before introducing fish of
a different species. The second site at which blockage can occur is that of
electron transfer involving the b
cytochromes, coenzyme Q, and cytochrome c1.
The classic inhibitor associated with this blockage is the antibiotic
antimycinA (Figure 20.21). More recently developed inhibitors that are active
in this part of the electron transport chain include myxothiazol and 5-n-undecyl-6-hydroxy-4,7-dioxobenzothiazol
(UHDBT). These compounds played a role in establishing the existence of the Q
cycle. The third site subject to blockage is the transfer of electrons from the
cytochrome aa3 complex to
oxygen. Several potent inhibitors operate at this site (Figure 20.22), such as
cyanide (CN–, azide (N3–), and carbon monoxide
(CO). Note that each of the three sites of action of respiratory inhibitors
corresponds to one of the respiratory complexes. Research is continuing with
some of the more recently developed inhibitors; the goal of additional work is
to elucidate more of the details of the electron transport process.
Summary
Respiratory inhibitors block the electron transport chain at sites
that cor-respond to each of the respiratory complexes.
Experiments on these substances, many of which are highly toxic,
were used to determine the path of electrons in respiration.
Related Topics