Chapter: Mechanical : Metrology and Measurements : Measurement of Power, Flow and Temperature Related Properties
Temperature Measurement
TEMPERATURE MEASUREMENT
Temperature is one of the most measured
physical parameters in science and technology; typically for process thermal
monitoring and control. There are many ways to measure temperature, using
various principles.
Four
of the most common are:
·
Mechanical (liquid-in-glass
thermometers, bimetallic strips, etc.)
·
Thermojunctive (thermocouples)
·
Thermoresistive (RTDs and thermistors)
·
Radiative (infrared and optical
pyrometers)
Mechanical Temperature
Measuring Devices
A change in temperature causes some kind of
mechanical motion, typically due to the fact that most materials expand with a
rise in temperature. Mechanical thermometers can be constructed that use
liquids, solids, or even gases as the temperature-sensitive material. The
mechanical motion is read on a physical scale to infer the temperature.
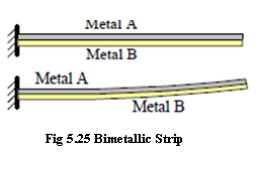
Bimetallic strip
thermometer
·
Two dissimilar metals are bonded
together into what is called a bimetallic strip, as sketched to the right.
·
Suppose metal A has a smaller
coefficient of thermal expansion than does metal B. As temperature increases,
metal B expands more than does metal A, causing the bimetallic strip to curl
upwards as sketched.
·
One common application of bimetallic
strips is in home thermostats, where a bimetallic strip is used as the arm of a
switch between electrical contacts. As the room temperature changes, the
bimetallic strip bends as discussed above. When the bimetallic strip bends far
enough, it makes contact with electrical leads that turn the heat or air
conditioning on or off.
·
Another application is in circuit
breakers High temperature indicates over-current, which shuts off the circuit.
·
Another common application is for use as
oven, wood burner, or gas grill thermometers. These thermometers consist of a
bimetallic strip wound up in a spiral, attached to a dial that is calibrated
into a temperature scale.
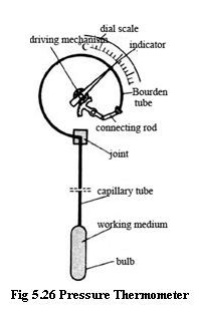
Pressure thermometer
·
A pressure thermometer, while still
considered mechanical, operates by the expansion of a gas instead of a liquid
or solid. There are also pressure thermometers that use a liquid instead of a
gas
· Suppose
the gas inside the bulb and tube can be considered an ideal gas. The ideal gas
law is PV = mRT, where P is the pressure, V is the volume of the gas, m is the
mass of the gas, R is the gas constant
for the specific gas (not the universal gas constant), and T is the absolute
temperature of the gas.
· • Specific gas constant R is a constant.
The bulb and tube are of constant volume, so V is a constant. Also, the mass m
of gas in the sealed bulb and tube must be constant (conservation of mass).
· • A pressure thermometer therefore
measures temperature indirectly by measuring pressure.
· • The gage is a pressure gage, but is
typically calibrated in units of temperature instead.
· • A common application of this type of
thermometer is measurement of outside temperature from the inside of a
building. The bulb is placed outside, with the tube running through the wall
into the inside.
·
• The
gauge is on the inside. As T increases outside, the bulb temperature causes a
corresponding increase in pressure, which is read as a temperature increase on
the gauge.
Related Topics