Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Gene Therapy
Systems for Pharmacological Regulation of Gene Expression
Systems for Pharmacological Regulation of Gene Expression
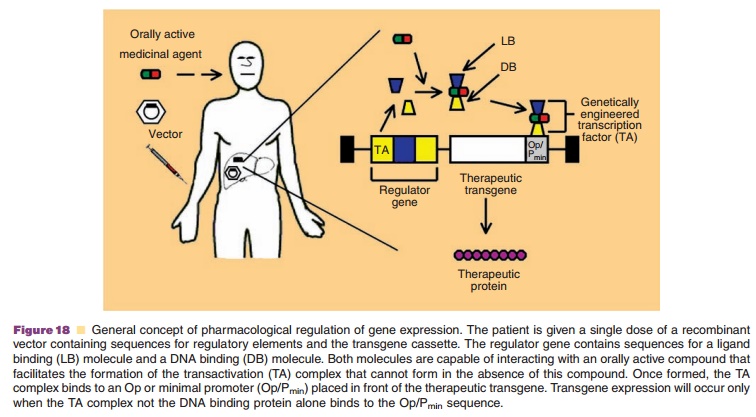
Strict regulation of gene expression in a consistent manner when and
where the transgene is needed is a requirement for successful clinical
implementation of gene therapy. Several systems consisting of a chimeric
promoter containing a specific tandem operator (Op) placed adjacent to a
minimal promoter (Pmin) that drives expression of the
therapeutic transgene are under development (Fig. 18). The chimeric promoter is
activated by binding of a transactivator (TA) complex to the Op. The TA complex
consists of a DNA binding domain (DB) and a ligand binding (LB) domain, capable
of interacting with an orally active compound. The compound facilitates
formation of the TA complex and dictates gene expression or repres-sion.
Regulated gene expression systems currently under development are responsive to
small-molecule drugs such as antibiotics, steroids, immunosuppres-sants and
their derivatives (Goverdhana, 2005). In an optimized regulated gene expression
system, each component must be non-immunogenic, safe and well tolerated.
Transgene expression must be undetectable when repressed and highest when
induced. The inducer is reversible and compact in size for
efficientincorporation in gene delivery systems. The regulat-ing compound
should be non-toxic, able to distribute to the target tissue and have a
half-life of a few hours so that gene expression can be altered quickly. These
issues are common to the development of traditional pharmaceutical compounds
and are areas in which the pharmacist and the pharmaceutical scientist can make
significant contributions.
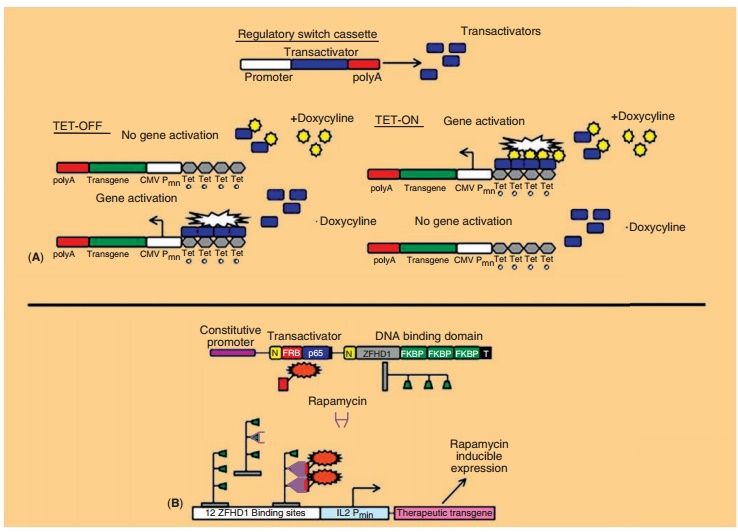
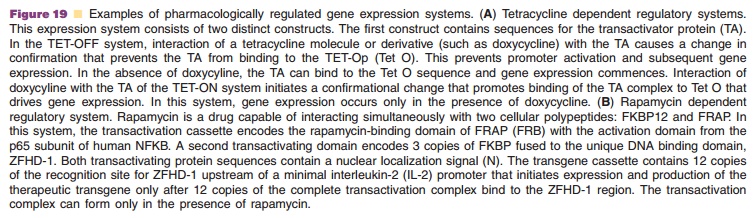
The most widely tested regulated gene expression systems are the
TET-ON/TET-OFF and Rapamycin/ Rapalog dimerizer systems (Fig. 19) (Goverdhana,
2005; Vilaboa, 2006). They have been engineered primarily in recombinant
adenovirus, AAV and retrovirus vectors (Goverdhana, 2005; Weber, 2006).
Although all of the systems currently under investigation are in pre-clinical
testing, they are on the brink of entering clinical trials. While these systems
involve dosing and control of a single therapeutic transgene, development of
elaborate semi-synthetic gene networks, capable of interacting seamlessly with
cellular processes to produce self-sustained, feedback-controlled and
physiologically triggered transgene expression is underway. This approach, if
successful in the clinic, will most likely become the premiere technology of
molecular medicine.
The majority of pharmacologically regulated gene expression systems have
been tested in animal models of cancer and hormone-related diseases. One
approach for the treatment of certain malignancies is the adoptive transfer of
T-cells. A problem with this method, however, is that the cells may recognize
both normal and malignant tissue and cause fatal graft-
Related Topics