Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Gene Therapy
General Anatomy and Production of a Gene Transfer Vector
General Anatomy and Production of a Gene Transfer Vector
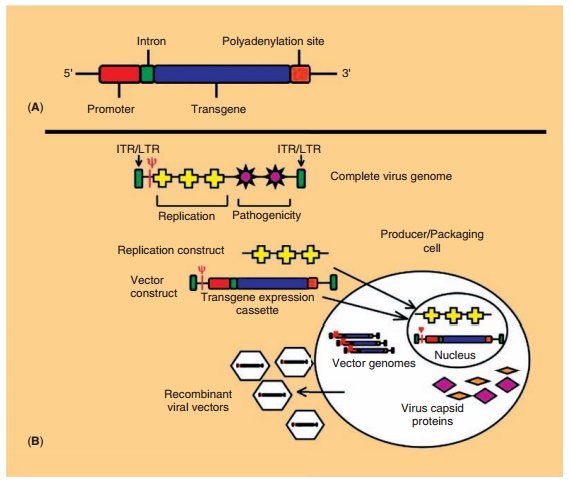
A gene-based medicine typically consists of an expression cassette made
of cDNA flanked by apromoter on the 50 side and
a transcription stop and polyadenylation site on the 30 side (Fig. 4A). This is incorporated into a DNA plasmid or a
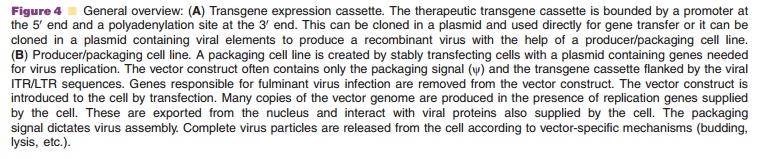
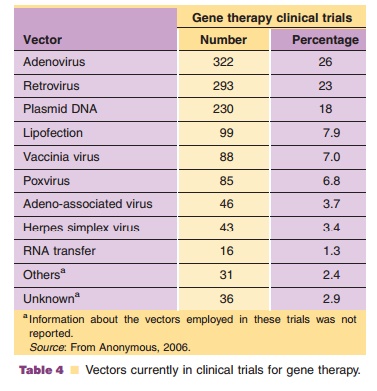
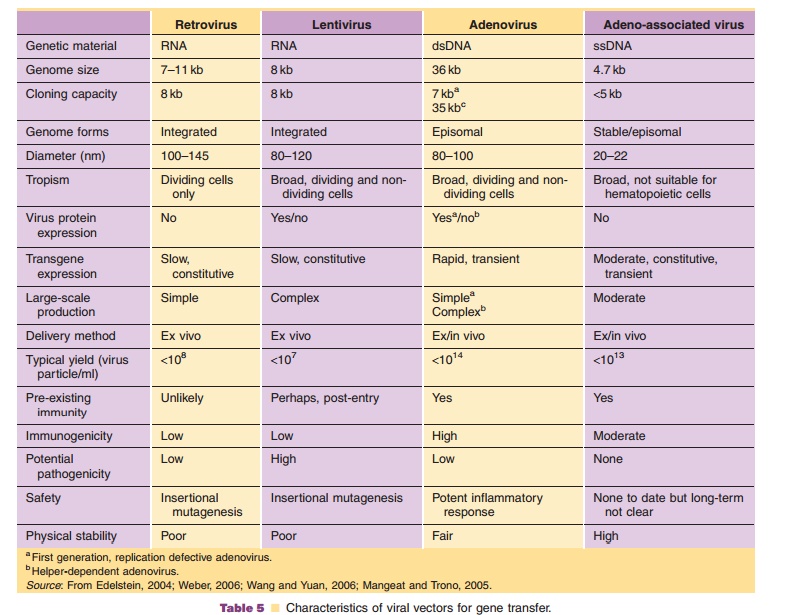
recombinant virus, based upon therapeutic requirements (Tables 4 and 5). The
genes responsible for the pathogenicity of viral vectors are removed and
replaced with the expression cassette in order to limit virus reproduc-tion and
fulminant disease. In many vectors, all that remains of the original virus
genome are long terminal repeats (LTRs), 5 and 3 terminal regions of the virus
that control transcription for RNA viruses or inverted terminal repeats (ITRs),
identical but oppo-sitely oriented sequences that drive DNA replication and
stabilize the genome of DNA viruses. The packaging signal (y), responsible for virus assembly, is also kept intact. Genes for
replication are supplied in trans by a producer/packaging cell line
for large-scale production (Fig. 4B). Virus biology will dictate the manner in
which the recombinant vector is produced. Careful thought must go into the
design of a packaging cell line in order to minimize overlap between sequences
in the virus genome and the cell that dictate replication and/or pathogenesis.
If sub-stantial overlap exists, these sequences can inadver-tently be
incorporated in the recombinant virus by homologous recombination. Replication
competent (pathogenic) virus particles will then be produced with replication
deficient particles. Preparations are often screened for replication competent
virus (RCV) prior to clinical use. After production and harvest from a
packaging cell line, recombinant vectors are purified, quantified and/or
titered. Traditionally, purification strategies have relied upon density
gradient ultracentrifugation to separate the vector from cellular proteins.
This process is laborious, difficult to scale up and can reduce the effective
titer of stock preparations by disrupting vector structure (Shamlou, 2003;
Burova, 2005). Advances in column chromatography have mitigated these issues
for many vectors, allowing them to be grown and purified to high concentrations
needed for human use.
Given the diversity of diseases suitable for gene therapy, it is
apparent that there can be no single vector that is appropriate for all gene
transfer applications. Thus, selection of an appropriate vector for gene
delivery requires careful consideration of a number of factors including: (a) size limitations for transgene cassettes, (b) transduction efficiency in therapeutic target (ability to infect
dividing and/or non-dividing cells, appropriate receptors present on target
cells), (c) duration of gene expression required for treatment (long-term vs.
transient expression, integrating vs. non-integrating vectors), (d) necessity of temporal gene expression (inducible expression vs.
constitutive expression), (e) maximum threshold of
vector-induced immune response and toxicity
Related Topics