Chapter: Organic Chemistry: Carboxylic acids and carboxylic acid derivatives
Structure and properties of Carboxylic acids and carboxylic acid derivatives
STRUCTURE AND PROPERTIES
Key Notes
Structure
There
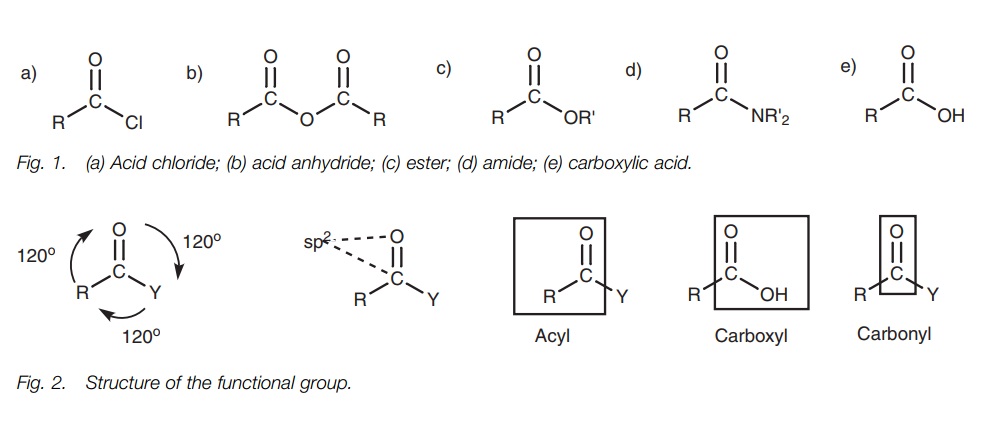
are four common carboxylic acid derivaties derived from a parent carboxylic
acid – acid chlorides, acid anhydrides, esters, and amides. Car-boxylic acids
and their derivatives contain ansp2
hybridized carbonyl group linked to a group Y where the atom directly attached
to the carbonyl group is a heteroatom (Cl, O or N). The functional groups are
planar with bond angles of 120°.
Bonding
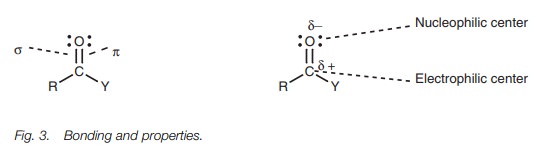
The
carbonyl group is made up of a strong σ bond and a weaker π bond. The carbonyl group is polarized such
that the oxygen acts as a nucleophilic center and the carbon acts as an
electrophilic center.
Properties
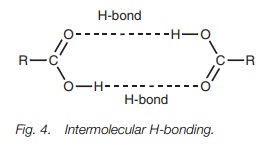
Carboxylic
acids are polar and can take part in hydrogen bonding. They are soluble in
water and have high boiling points. Carboxylic acids are weak acids in aqueous
solution and form water soluble salts when treated with a base. Primary and
secondary amides participate in hydrogen bonding and have higher boiling points
than comparable aldehydes or ketones. Acid chlorides, acid anhydrides, esters,
and tertiary amides are polar but are not capable of hydrogen bonding. Their
boiling points are similar to aldehydes and ketones of similar molecular
weight.
Reactions
Carboxylic
acids and acid derivatives undergo nucleophilic substitutions.
Spectroscopic analysis
The
presence of a carboxylic acid or a carboxylic acid derivative can be
demonstrated by spectroscopy. IR spectroscopy shows strong absorptions for
carbonyl stretching. The position of the absorption is characteristic of
different acid derivatives. Quaternary signals for the carbonyl carbon occur in
the 13C nmr spectrum and also occur in characteristic regions for
each acid derivative. It is important to consider all other lines of evidence
when interpreting spectra. This includes elemental analysis, molecular weight
and molecular formula, as well as supporting evidence in various spectra.
Structure
Carboxylic acid derivatives are structures
derived from a parent carboxylic acid structure. There
are four common
types of acid
derivative – acid
chlorides, acid anhydrides,
esters, and amides (Fig. 1). These
functional groups contain a carbonyl group (C=O) where both atoms are sp2 hybridized (Fig. 2). The carbonyl group along with
the two neighboring atoms is planar with bond angles of 120°.
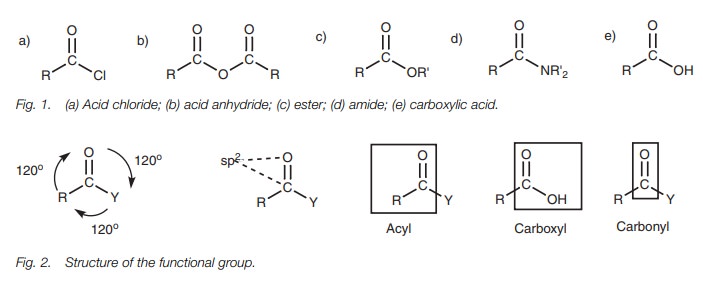
The carbonyl group along with the attached
carbon chain is called an acyl
group.
Carboxylic acids and carboxylic acid
derivatives differ in what is attached to the acyl group (i.e. Y = Cl, OCOR,
OR, NR2, or OH). Note that in all these cases, the atom in Y which
is directly attached to the carbonyl group is a heteroatom (Cl, O, or N). This
distinguishes carboxylic acids and their derivatives from aldehydes and ketones
where the corresponding
atom is hydrogen
or carbon. This
is important with respect to the sort of reactions which carboxylic
acids and their derivatives undergo. The carboxylic acid group (COOH) is often
referred to as a carboxyl group.
Bonding
The bonds in the carbonyl C O group are made up
of a strong σ bond
and a weaker π bond (Fig. 3). Since oxygen is more
electronegative than carbon, the carbonyl group is polarized such that the
oxygen is slightly negative and the carbon is slightly positive. This means
that oxygen can act as a nucleophilic center and carbon can act as an
electrophilic center.
Properties
Carboxylic acids and their derivatives are
polar molecules due to the polar carbonyl group and the presence of a
heteroatom in the group Y. Carboxylic acids can associate with each other as
dimers (Fig. 4) through the formation
of two intermolecular hydrogen bonds which means that carboxylic acids have
higher boiling points than alcohols of comparable molecular weight. It also
means that low molecular weight carboxylic acids are soluble in water. However,
as the molecular weight of the carboxylic acid increases, the hydrophobic
character of the alkyl portion eventually outweighs the polar character of the
functional group such that higher molecular weight carboxylic acids are
insoluble in water.
Primary amides and secondary amides also have a
hydrogen capable of hydro-gen bonding (i.e. RCONHR’, RCONH2),
resulting in higher boiling points for
Acid chlorides, acid anhydrides, esters,
and tertiary amides are polar, but lack a hydrogen atom capable of
participating in hydrogen bonding. As a result, they have lower boiling points
than carboxylic acids or alcohols of similar molec-ular weight, and similar
boiling points to comparable aldehydes and ketones.
Carboxylic acids are weak acids in aqueous
solution, forming an equilibrium between the free acid and the carboxylate ion.
In the presence of a base such as sodium hydroxide or sodium hydrogen
carbonate, they ionize to form water-soluble salts and this provides a method
of separating carboxylic acids from other organic compounds.
Reactions
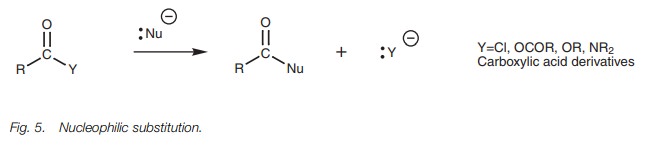
Carboxylic acids and carboxylic acid
derivatives commonly react with nucleophiles in a reaction known as nucleophilic substitution (Fig. 5). The reaction involves
replacement of one nucleophile with another. Nucleophilic substitution is
possible because the displaced nucleophile contains an electronegative heteroatom
(Cl, O, or N) which is capable of stabilizing a negative charge.
Spectroscopic analysis
The presence of a carboxylic acid can be
demonstrated by spectroscopy. The IR spectrum of an aliphatic carboxylic acid
shows a very broad O–H stretching absorption covering the range 3200–2500 cm−1, as well as a strong absorption for carbonyl
stretching in the range 1725–1700 cm−1. Less obvious absorptions may be observed in
the fingerprint region for O–H bending and C–O stretching (1320–1220 cm−1). If the carboxylic acid is conjugated to a
double bond or an aromatic ring, the carbonyl absorption occurs at relatively
lower wavenumbers (in the ranges 1715–1690 and 1700–1680 cm−1 respectively). The 1H nmr spectrum
of a carboxylic acid will contain a singlet for the acidic proton at a high
chemical shift (9–15 ppm) which is D2O exchangeable, while the 13C
nmr spectrum shows a quaternary signal for the carbonyl carbon (166–181 ppm).
In the mass spectrum, fragmentation ions may be observed due to loss of OH
(M-17) as well as loss of CO2H (M-45). A fragmentation ion due to
[CO2H]+ may be present at m/e 45.
Distinguishing a carboxylic acid derivative
from a carboxylic acid is easy since the 1H nmr spectrum of the
former will lack a signal for the acidic proton. More- over, the characteristic
broad O–H stretching absorption will be absent from the IR spectrum.
Distinguishing between different carboxylic acid derivatives is less
straightforward, but it is still feasible. All the acid derivatives show a
carbonyl stretching absorption in the IR spectrum. The position of this
absorption can indicate the group present (e.g. acid chloride 1815–1790 cm−1; acid anhydride 1850–1710 cm−1; ester 1750–1735 cm−1; amide 1700–1630 cm−1). Unfortunately, there is an overlap between
the regions, which can sometimes make the assignment difficult. This problem is
compounded if the functional group is conjugated to an aromatic ring or double
bond since this lowers the possible frequency range and increases possible
overlap. For example, the carbonyl absorption for an aliphatic ester is in the
range 1750–1735 cm−1, whereas the absorption range for an α,β-unsaturated ester and an aromatic ester are both within the range
1730–1715 cm−1. For that reason, it is important to look at
supporting evidence before assuming the presence of a particular functional
group. For example, there are other characteristic absorptions in the IR
spectrum that can help to distinguish one acid derivative from another. Acid
anhydrides are distinctive in having two car-bonyl absorptions and may also
have two visible C–O stretching absorptions in the range 1300–1050 cm−1. Esters may also have two visible C–O
stretching absorp-tions in the same region but will only have one carbonyl
absorption. Primary and secondary amides have N–H stretching absorptions
(3500–3400 cm−1 and 3460–3400 cm−1 respectively) as well as N–H bending
absorptions (1640– 1600 cm−1 and 1570–1510 cm−1 respectively). Tertiary amides naturally lack
these absorptions.
The 13C nmr spectra for acid
derivatives will contain a quaternary signal for the carbonyl carbon. For an
aliphatic ester this appears at 169–176 ppm while conju-gated esters have a
signal at lower chemical shift (164–169 ppm). The carbonyl signal for acid
anhydrides occurs in the range 163–175 ppm. For amides it occurs at 162–179
ppm, and for acid chlorides it is present at 167–172 ppm.
If a mass spectrum and elemental analysis have
been taken, the molecular weight and molecular formula will be known. This can
establish whether a particular acid derivative is present or not. For example,
the lack of chlorine or nitrogen in the formula clearly rules out the
possibility of an acid chloride or amide; the presence of only one oxygen rules
out the possibility of an ester or acid anhydride. This may seem obvious but it
is surprising how often this information is ignored when students attempt to
interpret IR spectra.
The chemical shifts of certain groups can give
indirect evidence of particular acid derivatives, both in the 1H and
13C nmr spectra. For example, the methyl group of a methyl alkanoate
appears at 3.7 ppm in the 1H spectrum and at 51–52 ppm in the carbon
spectrum. In contrast, the N-methyl
group of an amide occurs at 2.9 ppm (1H nmr) and 31–39 ppm (13C
nmr).
Related Topics