Chapter: Organic Chemistry: Aldehydes and ketones
Aldehydes and ketones: ╬▒,╬▓-Unsaturated aldehydes and ketones
╬▒,╬▓-UNSATURATED ALDEHYDES AND KETONES
Key Notes
Definition
╬▒,╬▓-Unsaturated aldehydes and ketones are
aldehydes and ketones whichare conjugated with a double bond.
Nucleophilic and electrophilic centers
The
carbonyl oxygen of an ╬▒,╬▓-unsaturated aldehyde or ketone is a
nucle-ophilic center. The carbonyl carbon and the ╬▓-carbon are electrophilic centers. Nucleophilic
addition can take place at either the carbonyl carbon (1,2-addition), or the ╬▓-carbon (1,4- or conjugate
addition).
1,2-Addition
1,2-Addition
to ╬▒,╬▓-unsaturated aldehydes and
ketones takes place with Grignard reagents and organolithium reagents.
1,4-Addition
1,4-Addition
to ╬▒,╬▓-unsaturated aldehydes and
ketones takes place with organocuprate reagents, amines and the cyanide ion.
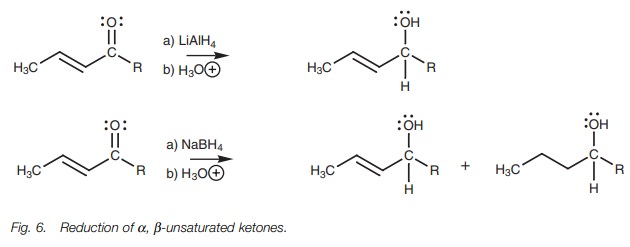
Reduction
╬▒,╬▓-unsaturated ketones are reduced to allylic
alcohols with lithiumaluminum hydride.
Definition
╬▒,╬▓-Unsaturatedaldehydes and ketones are aldehydes
and ketones which are conjugated with a double bond. The ╬▒-position is defined as the carbon atom next to the carbonyl group,
while the ╬▓-position is the carbon atom two bonds removed
(Fig. 1).
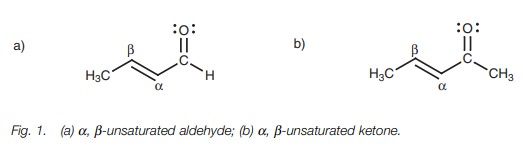
Nucleophilic and electrophilic centers
The carbonyl group of ╬▒,╬▓-unsaturated aldehydes and ketones consists of a nucleophilic oxygen and an electrophilic carbon. However, ╬▒,╬▓-unsaturated aldehydes and ketones also have another electrophilic carbon ŌĆō the ╬▓-carbon. This is due to the influence of the electronegative oxygen which can result in the resonance shown (Fig. 2).
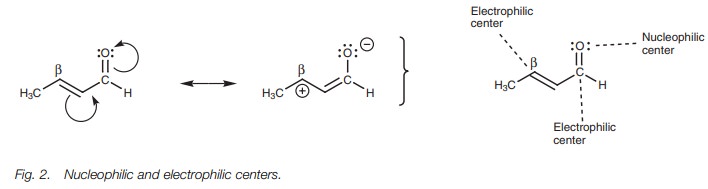
Since two electrophilic centers are present, there are two places where a nucleophile can react. In both
situations, an addition reaction takes place. If the nucleophile reacts with
the carbonyl carbon, this is a normal nucleophilic addition to an aldehyde or
ketone and is called a 1,2-nucleophilicaddition.
If the nucleophile adds to the╬▓-carbon, this is known as a 1,4-nucleophilic addition or a
conjugate addition.
1,2-Addition
The mechanism of 1,2-nucleophilic addition is
the same mechanism already described. It is found that Grignard reagents and
organolithium reagents will react with ╬▒,╬▓-unsaturated aldehydes and ketones in this way
and do not attack the ╬▓-position (Fig.
3).

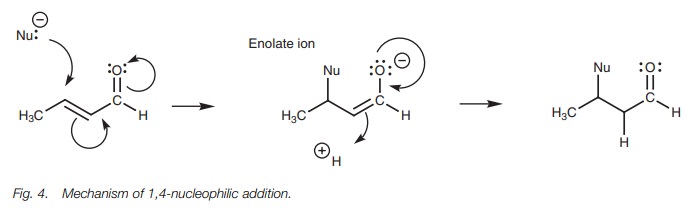
1,4-Addition
The mechanism for 1,4-addition involves two
stages (Fig. 4). In the first stage,
the nucleophile uses a lone pair of electrons to form a bond to the ╬▓-carbon. At the same time, the C=C ŽĆ bond breaks and both electrons are used to
form a new ŽĆ bond to the carbonyl carbon. This in turn
forces the carbonyl ŽĆ bond to break with both of the electrons
involved moving onto the oxygen as a third lone pair of electrons. The
resulting intermediate is an enolate ion. Aqueous acid is now added to the
reaction mixture. The carbonyl ŽĆ bond is reformed, which forces open the C=C ŽĆ bond. These electrons are
now used to form a Žā bond to
a proton at the ╬▒ carbon.
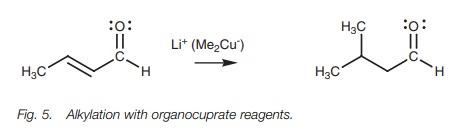
Conjugate addition reactions can be carried out
with amines, or a cyanide ion. Alkyl groups can also be added to the ╬▓-position by using organocuprate reagents. A large variety of organocuprate
reagents can be prepared allow-ing the addition of primary, secondary and
tertiary alkyl groups, aryl groups, and alkenyl groups.
Reduction
The reduction of ╬▒,╬▓-unsaturated ketones to allylic alcohols is
best carried out using lithium aluminum hydride under carefully controlled
conditions (Fig. 6). With sodium
borohydride, some reduction of the alkene also takes place.
Related Topics