Chapter: Organic Chemistry: Aldehydes and ketones
Nucleophilic addition ŌĆō charged nucleophiles
NUCLEOPHILIC ADDITION ŌĆō CHARGED NUCLEOPHILES
Key Notes
Carbanion addition
Grignard
reagents (RMgX) and organolithium reagents (RLi) are used as the source of
carbanions. The reaction mechanism involves nucleophilic addi- tion of the
carbanion to the aldehyde or ketone to form a negatively charged intermediate.
Addition of acid completes the reaction. Both reactions are important because
they involve CŌĆōC bond formation allowing the synthesis of complex molecules
from simple starting materials. Primary alcohols are obtained from
formaldehyde, secondary alcohols
from aldehydes and tertiary alcohols from ketones.
Hydride addition
Lithium
aluminum hydride (LiAlH4) and sodium borohydride (NaBH4) are reducing agents
and the overall reaction corresponds to the nucleophilic addition of a hydride
ion (H: ŌĆō). The reaction is a functional group transfor- mation where primary
alcohols are obtained from aldehydes and secondary alcohols are obtained from
ketones.
Cyanide addition
Reaction
of aldehydes and ketones with HCN and KCN produce cyano- hydrins. The cyanide
ion is the nucleophile and adds to the electrophilic carbonyl carbon.
Bisulfite addition
The
bisulfite ion is a weakly nucleophilic anion which will only react with
aldehydes and methyl ketones. The product is a water-soluble salt and so the
reaction can be used to separate aldehydes and methyl ketones from larger
ketones or from other water-insoluble compounds. The aldehyde and methyl ketone
can be recovered by treating the salt with acid or base.
Aldol reaction
The
Aldol reaction involves the nucleophilic addition of enolate ions to aldehydes
and ketones to form ╬▓-hydroxycarbonyl compounds.
Carbanion addition
Carbanions are extremely reactive species and
do not occur in isolation. However, there are two reagents which can supply the
equivalent of a carbanion. These are Grignard reagents and organolithium
reagents. We shall look first of all at the reaction of a Grignard reagent with
aldehydes and ketones (Fig. 1).
The
Grignard reagent in
this reaction is
called methyl magnesium
iodide
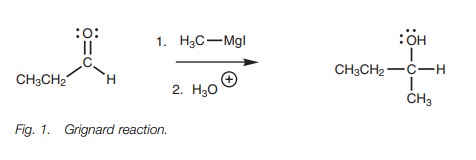
In reality, the methyl carbanion is never present as a
separate ion, but the reaction proceeds as if it were. The methyl carbanion is
the nucleophile in this reaction and the nucleophilic center is the negatively
charged carbon atom. The aldehyde is the electrophile. Its electrophilic center
is the carbonyl carbon atom since it is electron deficient.
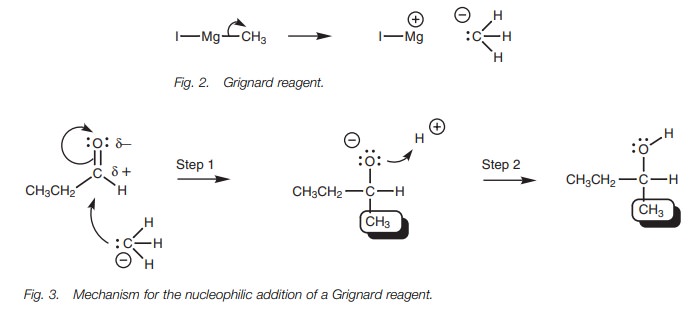
The carbanion uses its lone pair of electrons
to form a bond to the electrophilic carbonyl carbon (Fig. 3). At the same time, the relatively weak ŽĆ bond of the car-bonyl group breaks and both electrons move to the
oxygen to give it a third lone pair of electrons and a negative charge (Step
1). The reaction stops at this stage, since the negatively charged oxygen is
complexed with magnesium which acts as a counterion (not shown). Aqueous acid
is now added to provide an electrophile in the shape of a proton. The
intermediate is negatively charged and can act as a nucleophile/base. A lone
pair of electrons on the negatively charged oxygen is used to form a bond to
the proton and the final product is obtained (Step 2).
The reaction of aldehydes and ketones with
Grignard reagents is a useful method of synthesizing primary, secondary, and
tertiary alcohols (Fig. 4). Primary
alcohols can be obtained from formaldehyde, secondary alcohols can be obtained
from aldehydes, and tertiary alcohols can be obtained from ketones. The
reaction involves the formation of a carbonŌĆōcarbon bond and so this is an
important way of building up complex organic structures from simple starting
materials.
The Grignard reagent itself is synthesized from
an alkyl halide and a large variety of reagents are possible.
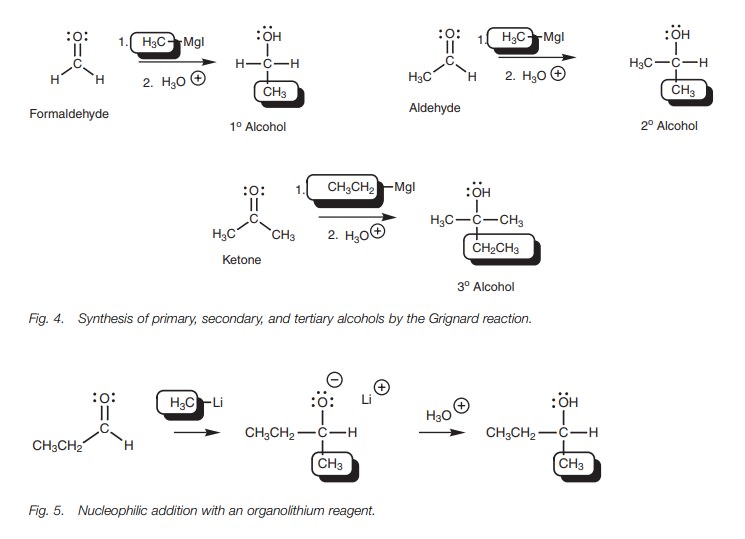
Organolithium reagents such as CH3Li can also be used to provide the nucleophilic carbanion and the reaction mechanism is exactly the same as that described for the Grignard reaction (Fig. 5).
Hydride addition
Reducing agents such as sodium borohydride
(NaBH4) and lithium aluminum hydride (LiAlH4) react with
aldehydes and ketones as if they are providing a hydride ion (:HŌĆō; Fig. 6). This species is not present as
such and the reaction mechanism is more complex. However, we can explain the
reaction by viewing these reagents as hydride equivalents (:HŌĆō). The
overall reaction is an example of a functional group transformation since the
carbon skeleton is unaffected. Aldehydes are converted to primary alcohols and
ketones are converted to secondary alcohols.
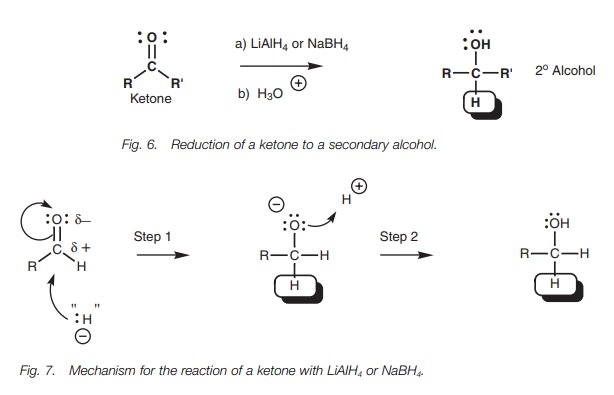
The mechanism of the reaction is the same as that described above for the Grignard reaction (Fig. 7). The hydride ion equivalent adds to the carbonyl group and a negatively charged intermediate is obtained which is complexed as a lithium salt (Step 1). Subsequent treatment with acid gives the final product (Step 2). It should be emphasized again that the mechanism is actually more complex than this because the hydride ion is too reactive to exist in isolation.
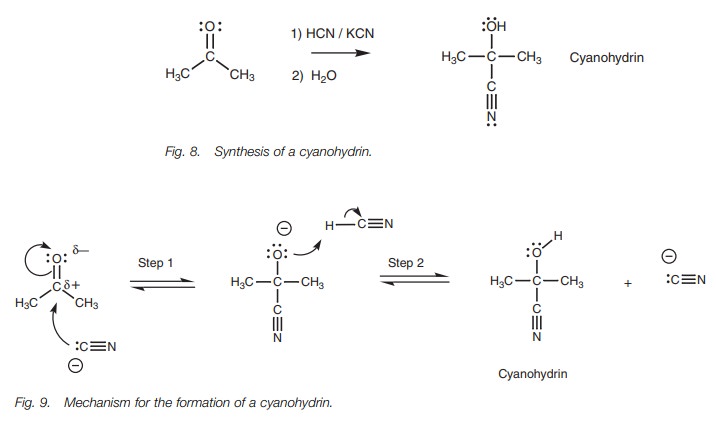
Cyanide addition
Nucleophilic addition of a cyanide ion to an aldehyde or ketone gives a cyanohydrin (Fig. 8). In the reaction, there is a catalytic amount of potassiumcyanide present and this supplies the attacking nucleophile in the form of the cyanide ion (CNŌĆō). The nucleophilic center of the nitrile group is the carbon atom since this is the atom with the negative charge. The carbon atom uses its lone pair of electrons to form a new bond to the electrophilic carbon of the carbonyl group (Fig. 9). As this new bond forms, the relatively weak ŽĆ bond of the carbonyl group breaks and the two electrons making up that bond move onto the oxygen to give it a third lone pair of electrons and a negative charge (Step 1). The intermediate formed can now act as a nucleophile/base since it is negatively charged and it reacts with the acidic hydrogen of HCN. A lone pair of electrons from oxygen is used to form a bond to the acidic proton and the HŌĆōCN Žā bond is broken at the same time such that these electrons move onto the neighboring carbon to give it a lone pair of electrons and a negative charge (Step 2). The products are the cyanohydrin and the cyanide ion. Note that a cyanide ion started the reaction and a cyanide ion is regenerated. Therefore, only a catalytic amount of cyanide ion is required to start the reaction and once the reaction has taken place, a cyanide ion is regenerated to continue the reaction with another molecule of ketone.
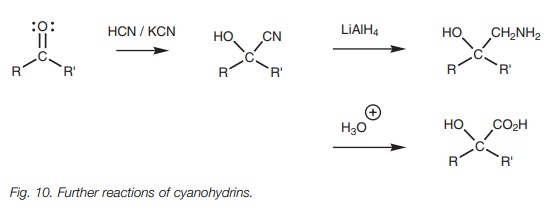
Cyanohydrins are useful in synthesis because
the cyanide group can be converted to an amine or to a carboxylic acid.
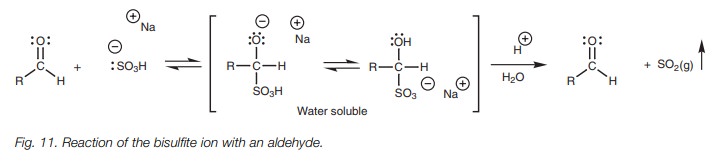
Bisulfite addition
The reaction of an aldehyde or a methyl ketone with sodium bisulfite (NaHSO3) involves nucleophilic addition of a bisulfite ion (ŌĆō:SO3H) to the carbonyl group to give a water soluble salt (Fig. 11). The bisulfite ion is a relatively weak nucleophile compared to other charged nucleophiles and so only the most reactive carbonyl compounds will react. Larger ketones do not react since larger alkyl groups hinder attack. The reaction is also reversible and so it is a useful method of separating aldehydes and methyl ketones from other ketones or from other organic molecules. This is usually done during an experimental work up where the products of the reaction are dissolved in a water immiscible organic solvent. Aqueous sodium bisulfite is then added and the mixture is shaken thoroughly in a separating funnel. Once the layers have separated, any aldehydes and methyl ketones will have undergone nucleophilic addition with the bisulfite solution and will be dissolved in the aqueous layer as the water soluble salt. The layers can now be separated. If the aldehyde or methyl ketone is desired, it can be recovered by adding acid or base to the aqueous layer which reverses the reaction and regenerates the carbonyl compound.
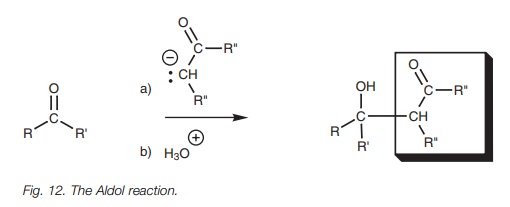
Aldol reaction
Another nucleophilic addition involving a
charged nucleophile is the Aldol reaction. This involves the nucleophilic
addition of enolate ions to aldehydes and ketones to form ╬▓-hydroxycarbonyl compounds (Fig.
12).
Related Topics