Chapter: Organic Chemistry: Aldehydes and ketones
Nucleophilic addition - nitrogen nucleophiles
NUCLEOPHILIC ADDITION ŌĆō NITROGEN NUCLEOPHILES
Key Notes
Imine formation
Primary
amines react with aldehydes and ketones to give an imine or Schiff base. The
reaction involves nucleophilic addition of the amine followed by elimination of
water. Acid catalysis aids the reaction, but too much acid hinders the reaction
by protonating the amine.
Enamine formation
Secondary
amines undergo the same type of mechanism as primary amines, but cannot give
imines as the final product. Instead, a proton is lost from a neighboring
carbon and functional groups called enamines are formed.
Oximes, semicarbazones and 2,4-dinitrophenylhydrazones
Aldehydes
and ketones can be converted to crystalline derivatives called oximes,
semicarbazones, and 2,4-dinitrophenylhydrazones. Such deriva-tives were useful
in the identification of liquid aldehydes and ketones.
Imine formation
The reaction of primary amines with aldehydes
and ketones do not give the products expected from nucleophilic addition alone.
This is because further reaction occurs once nucleophilic addition takes place.
As an example, we shall consider the reaction of acetaldehyde (ethanal) with a
primary amine ŌĆō methylamine (Fig. 1).
The product contains the methylamine skeleton, but unlike the previous
reactions there is no alcohol group and there is a double bond between the
carbon and the nitrogen. This product is called an imine or a Schiffbase.
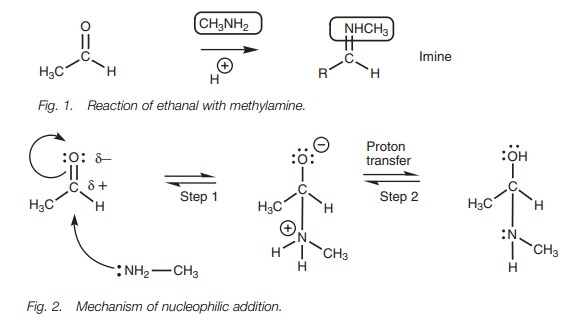
The first stage of the mechanism (Fig. 2) is a normal nucleophilic addition. The amine acts as the nucleophile and the nitrogen atom is the nucleophilic center. The nitrogen uses its lone pair of electrons to form a bond to the electrophilic carbonyl carbon. As this bond is being formed, the carbonyl ŽĆ bond breaks with both elec-trons moving onto the oxygen to give it a third lone pair of electrons and a nega-tive charge. The nitrogen also gains a positive charge, but both these charges can be neutralized by the transfer of a proton from the nitrogen to the oxygen (Step 2).
The oxygen uses up one of its lone pairs
to form the new OŌĆōH bond and the elec-trons in the NŌĆōH bond end up on the
nitrogen as a lone pair. An acid catalyst is present, but is not required for
this part of the mechanism ŌĆō nitrogen is a good nucleophile and although the
amine is neutral, it is sufficiently nucleophilic to attack the carbonyl group
without the need for acid catalysis. The intermediate obtained is the structure
one would expect from nucleophilic addition alone, but the reaction does not
stop there. The oxygen atom is now protonated by the acid catalyst and gains a
positive charge (Fig. 3, Step 3).
Since oxygen is electronega-tive, a positive charge is not favored and so there
is a strong drive to neutralize the charge. This can be done if the bond to
carbon breaks and the oxygen leaves as part of a water molecule. Therefore,
protonation has turned the oxygen into a good leaving group. The nitrogen helps
the departure of the water by using its lone pair of electrons to form a ŽĆ bond to the neighboring carbon atom and a pos-itive charged
intermediate is formed (Step 4). The water now acts as a nucleophile and
removes a proton from the nitrogen such that the nitrogenŌĆÖs lone pair is
restored and the positive charge is neutralized (Step 5).
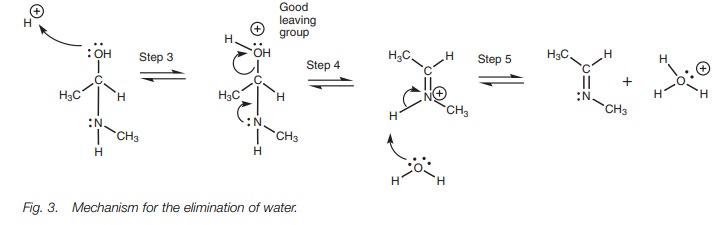
Overall, a molecule of water has been lost in
this second part of the mechanism.
Acid catalysis is important in creating a good
leaving group. If protonation did not occur, the leaving group would have to be
the hydroxide ion which is a more reactive molecule and a poorer leaving group.
Although acid catalysis is important to the
reaction mechanism, too much acid can actually hinder the reaction. This is
because a high acid concentration leads to protonation of the amine, and
prevents it from acting as a nucleophile.
Enamine formation
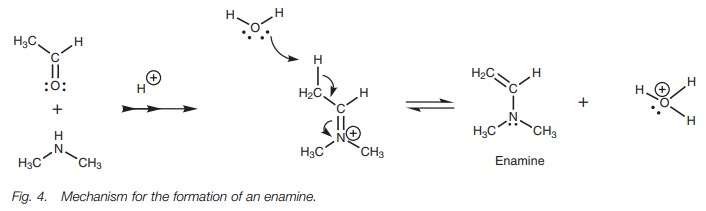
The reaction of carbonyl compounds with
secondary amines cannot give imines since there is no NH proton to be lost in
the final step of the mechanism. However, there is another way in which the
positive charge on the nitrogen can be neutralized. This involves loss of a
proton from a neighboring carbon atom (Fig.
4). Water acts as a base to remove the proton and the electrons which make
up the CŌĆōH Žā bond are used to form a new ŽĆ bond to the neighboring carbon. This in turn forces the existing ŽĆ bond between carbon and nitrogen to break such that both the ŽĆ electrons end up on the nitrogen atom as a lone pair, thus
neutralizing the charge. The final structure is known as an enamine and can
prove useful in organic synthesis.
Oximes, semicarbazones and 2,4- dinitrophenyl- hydrazones
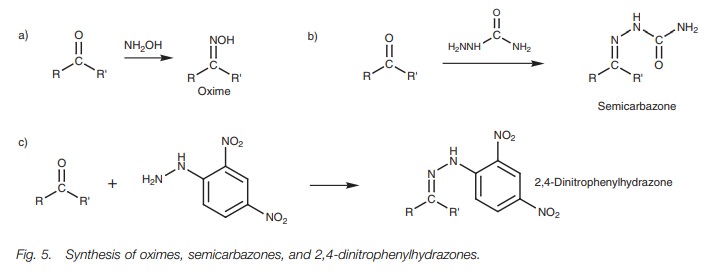
The
reaction of aldehydes
and ketones with
hydroxylamine (NH2OH),
semicarbazide (NH2NHCONH2) and
2,4-dinitrophenylhydrazine
takes place by the
same mechanism described for primary amines to give oximes, semi-
carbazones, and 2,4-dinitrophenylhydrazones, respectively
(Fig. 5). Thesecompounds were frequently synthesized in
order to identify a liquid aldehyde or ketone. The products are solid and
crystalline, and by measuring their melting points, the original aldehyde or
ketone could be identified by looking up melting point tables
of these derivatives.
Nowadays, it is
easier to identify
liquid aldehydes and ketones spectroscopically.
Related Topics