Chapter: Organic Chemistry: Aldehydes and ketones
Aldehydes and ketones: Reduction and oxidation
REDUCTION AND OXIDATION
Key Notes
Reduction to alcohols
Reduction
of an aldehyde with sodium borohydride or lithium aluminum hydride gives a
primary alcohol. Similar reduction of a ketone gives a secondary alcohol.
Reduction to alkanes
There
are three methods of deoxygenating aldehydes and ketones. The method used
depends on whether the compound is sensitive to acid or base. If sensitive to
acid, reduction is carried out under basic conditions by the Wolff–Kishner
reduction. If sensitive to base, the reaction is carried out under acid
conditions – the Clemmenson reduction. If sensitive to both acid and base, the
carbonyl group is converted to a dithioacetal or dithioketal then reduced with
Raney nickel.
Oxidation
Aldehydes
can be oxidized to carboxylic acids, but ketones are resistant to oxidation.
Reduction to alcohols
Aldehydes and ketones can be reduced to
alcohols with a hydride ion – provided by reducing reagents such as sodium
borohydride or lithium borohydride. Primary alcohols are obtained from
aldehydes, and secondary alcohols from ketones.
Reduction alkanes
Aldehydes and ketones can be reduced to alkanes
by three different methods which are complementary to each other. The Wolff–Kishner reduction is carried out
under basic conditions and is suitable for compounds that might be sensitive to
acid (Fig. 1). The reaction involves
the nucleophilic addition of hydrazine followed by elimination of water to form
a hydrazone. The mechanism is the same as that described for the synthesis of
2,4-dinitrophenylhydrazones.
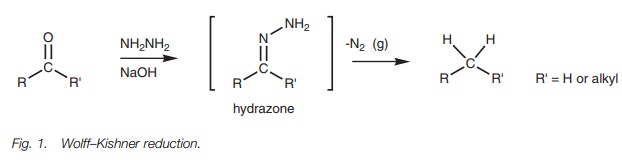
However, the simple hydrazone formed under
these reaction conditions spontaneously decomposes with the loss of nitrogen
gas.
The Clemmenson
reduction (Fig. 2) gives a similar product
but is carried out under acid conditions and so this is a suitable method for
compounds which are unstable to basic conditions.
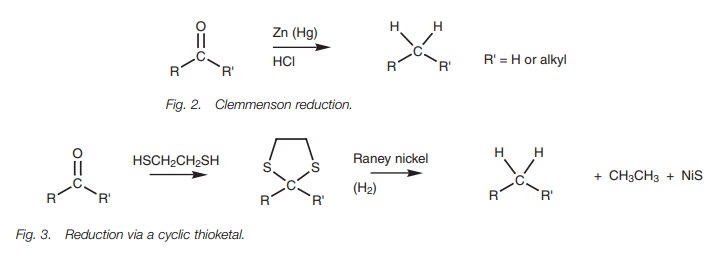
Compounds which are sensitive to both acid and
base can be reduced under neutral conditions by forming the thioacetal or
thioketal, then reducing with Raney nickel (Fig.
3).
Aromatic aldehydes and ketones can also be deoxygenated with hydrogen over a palladium charcoal catalyst. The reaction takes place because the aromatic ring activates the carbonyl group towards reduction. Aliphatic aldehydes and ketones are not reduced.
Oxidation
Ketones are resistant to oxidation whereas
aldehydes are easily oxidized. Treatment of an aldehyde with an oxidizing agent
results in the formation of a carboxylic acid (Fig. 4a). Some compounds may be sensitive to the acid conditions
used in this reaction and an alternative way of carrying out the oxidation is to
use a basic solution of silver oxide (Fig.
4b).
Both reactions involve the nucleophilic
addition of water to form a 1,1-diol or hydrate which is then oxidized in the
same way as an alcohol (Fig. 5);.

Related Topics