Chapter: Organic Chemistry: Aldehydes and ketones
Nucleophilic addition - oxygen and sulfur nucleophiles
NUCLEOPHILIC ADDITION ŌĆō OXYGEN AND SULFUR
NUCLEOPHILES
Key Notes
Acetal and ketal formation
The
reaction of aldehydes and ketones with two equivalents of an alcohol in the
presence of anhydrous acid as a catalyst results in the formation of acetals
and ketals respectively. The reaction involves nucleophilic addition of one
molecule of alcohol, elimination of water, then addition of a second molecule
of alcohol. The reaction is reversible and as a result acetals and ketals are
good protecting groups for aldehydes and ketones. The synthesis of the acetal
or ketal is carried out under anhydrous acid conditions while the reverse
reaction is carried out using aqueous acid. Cyclic acetals and ketals are
better protecting groups than acyclic ones.
Hemiacetals and hemiketals
Dissolving
aldehydes or ketones in alcohol results in an equilibrium between the carbonyl
compound and the hemiacetal/hemiketal. The reaction is slow and the equilibrium
favors the carbonyl compound. Most hemiacetals and hemiketals cannot be
isolated since they break back down to the original carbonyl compounds when the
solvent is removed. However, cyclic hemiacetals are important in sugar
chemistry.
Thioacetal and thioketal formation
Thioacetals
and thioketals can be synthesized by treating aldehydes and ketones with a thiol
or dithiol in the presence of an acid catalyst. These functional groups can
also be used to protect aldehydes and ketones but are more difficult to
hydrolyze. They can be useful in the reduction of aldehydes and ketones.
Acetal and ketal formation
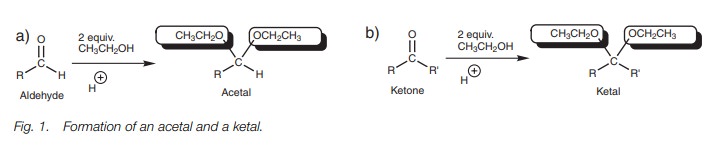
When an aldehyde or ketone is treated with an
excess of alcohol in the presence of an acid catalyst, two molecules of alcohol are added to the carbonyl compound to give
an acetal or a ketal respectively (Fig. 1).
The final product is tetrahedral.
The reaction mechanism involves the
nucleophilic addition of one molecule of alcohol to form a hemiacetal or
hemiketal. Elimination of water takes place to form an oxonium ion and a second
molecule of alcohol is then added (Fig. 2).
The mechanism is quite complex and we shall
look at it in detail by considering the reaction of methanol with acetaldehyde
(ethanal; Fig. 3). The aldehyde is
the electrophile and the electrophilic center is the carbonyl carbon. Methanol
is the nucleophile and the nucleophilic center is oxygen. However, methanol is
a relatively weak nucleophile. As a result, the carbonyl group has to be
activated by adding an acid catalyst if a reaction is to take place. The first
step of the mechanism involves the oxygen of the carbonyl group using a lone
pair of electrons to form a bond to a proton. This results in a charged
intermediate where the positive charge is shared between the carbon and oxygen
of the carbonyl group.
Protonation increases the electrophilicity of the carbonyl group, making the car-bonyl carbon even more electrophilic. As a result, it reacts better with the weakly nucleophilic alcohol. The alcoholic oxygen now uses one of its lone pairs of elec-trons to form a bond to the carbonyl carbon and the carbonyl ŽĆ bond breaks at the same time with the ŽĆ electrons moving onto the carbonyl oxygen and neutralizing the positive charge (Fig. 4). However, the alcoholic oxygen now has an unfavor-able positive charge (which explains why methanol is a weak nucleophile in the first place). This charge is easily lost if the attached proton is lost. Both electrons in the OŌĆōH Žā bond are captured by the oxygen to restore its second lone pair of electrons and neutralize the positive charge.
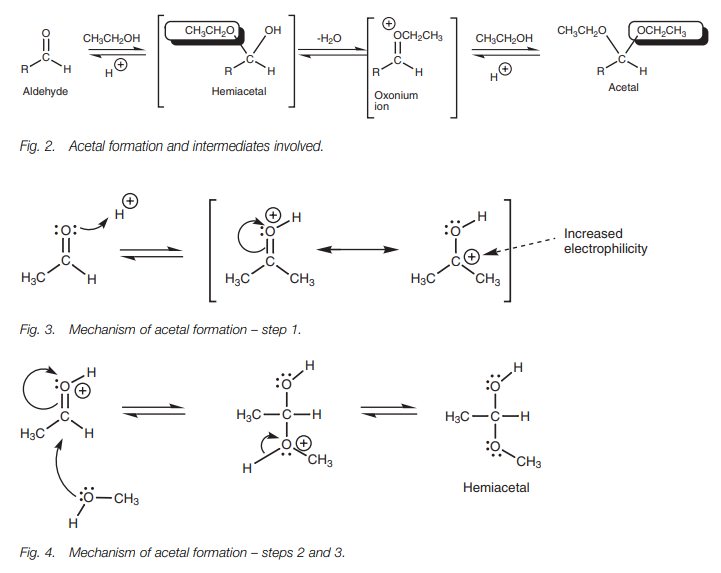
The intermediate formed from this first
nucleophilic addition is called a hemi-acetal.
If a ketone had been the starting material, the structure obtained wouldhave
been a hemiketal. Once the
hemiacetal is formed, it is protonated and water is eliminated by the same
mechanism described in the formation of imines
ŌĆō the only difference being that oxygen donates a lone pair of electrons
to force the removal of water rather than nitrogen (Fig. 5). The resulting oxonium ion is extremely electrophilic and a
second nucleophilic addition of alcohol takes place to give the acetal.
All the stages in this mechanism are reversible
and so it is possible to convert the acetal or ketal back to the original
carbonyl compound using water and an aqueous acid as catalyst. Since water is
added to the molecule in the reverse mechanism, this is a process called hydrolysis.
Acid acts as a catalyst both for the formation
and the hydrolysis of acetals and ketals, so how can one synthesize ketals and
acetals in good yield? The answer lies in the reaction conditions. When forming
acetals or ketals, the reaction is carried out in the absence of water using a
small amount of concentrated sulfuric acid or an organic acid such as para-toluenesulfonic acid. The yields
are further boosted if the water formed during the reaction is removed from the
reaction mixture.
In order to convert the acetal or ketal back to
the original carbonyl compound, an aqueous acid is used such that there is a
large excess of water present and the equilibrium is shifted towards the
carbonyl compounds.
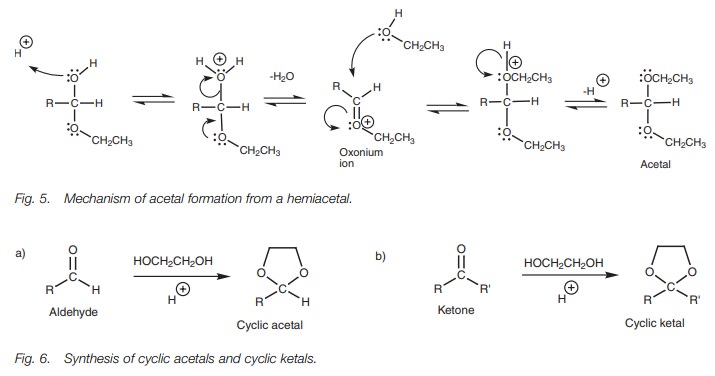
Both the synthesis and the hydrolysis of acetals and ketals can be carried out in high yield and so these functional groups are extremely good as protecting groups for aldehydes and ketones. Acetals and ketals are stable to nucleophiles and basic conditions and so the carbonyl group is ŌĆśdisguisedŌĆÖ and will not react with these reagents. Cyclic acetals and ketals are best used for the protection of aldehydes and ketones. These can be synthesized by using diols rather than alcohols (Fig. 6).
Hemiacetals and hemiketals
When aldehydes and ketones are dissolved in
alcohol without an acid catalyst being present, only the first part of the
above mechanism takes place with one alcohol molecule adding to the carbonyl
group.An equilibrium is set up between the carbonyl group and the hemiacetal or
hemiketal, with the equilibrium favoring the carbonyl compound (Fig. 7).
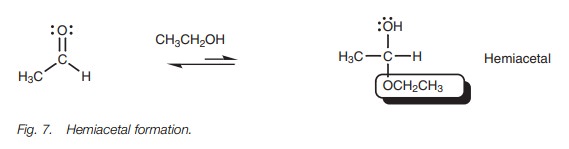
The reaction is not synthetically useful, since
it is not usually possible to isolate the products. If the solvent is removed,
the equilibrium is driven back to starting materials. However, cyclic
hemiacetals are important in the chemistry of sugars.
Thioacetal and thioketal formation
Thioacetals and thioketals are the sulfur
equivalents of acetals and ketals and are also prepared under acid conditions (Fig. 8). These can also be used to
protect aldehydes and ketones, but the hydrolysis of these groups is more
difficult. More importantly, the thioacetals and thioketals can be removed by
reduction and this provides a method of reducing aldehydes and ketones.
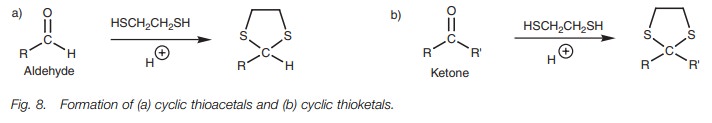
Related Topics