Chapter: Organic Chemistry: Aldehydes and ketones
Aldehydes and ketones: Reactions of enolate ions
REACTIONS OF ENOLATE IONS
Key Notes
Enolate ions
Enolate
ions are formed by treating aldehydes or ketones with a base. A proton has to
be present on the ╬▒-carbon.
Alkylation
Enolate
ions can be alkylated with an alkyl halide. O-Alkylation
and C-alky-lation are both possible,
but the latter is more likely and more useful. The reaction allows the
introduction of alkyl groups to the ╬▒-carbon of alde-hydes and ketones. If there are
two ╬▒-protons
present, two different alkyla-tions can be carried out in succession. ╬▓-Ketoesters are useful
starting materials since the ╬▒-protons are more acidic and the alkylation is
targeted to one position. The ester group is removed by decarboxylation.
Aldol reaction
The
Aldol reaction involves the dimerization of an aldehyde or a ketone. In the
presence of sodium hydroxide, aldehyde or ketone is converted to an enolate
ion, but not all the carbonyl molecules are converted and so the enolate ion
can undergo a nucleophilic addition on ŌĆśfreeŌĆÖ aldehyde or ketone. The product
is a ╬▓-hydroxyaldehyde
or ╬▓-hydroxyketone.
Aldehy-des react better than ketones in this reaction. If water is lost from
the Aldol adduct, an ╬▒,╬▓-unsaturated carbonyl structure is obtained.
Crossed Aldol reaction
The
crossed Aldol reaction links two different aldehyde structures. The reaction
works best if one of the aldehydes has no ╬▒-proton present and the other aldehyde is added
slowly to the reaction mixture to prevent self-condensation. If a ketone is
linked to an aldehyde, the reaction is known as the ClaisenŌĆōSchmidt reaction.
This works best if the aldehyde has no ╬▒-proton.
Enolate ions
Enolate ions are formed by treating aldehydes
or ketones with a base. An╬▒-proton has to be present. Enolate ions can
undergo a variety of important reactions including alkylation and the Aldol
reaction.
Alkylation
Treatment of an enolate ion with an alkyl halide results in a reaction known as alkylation (Fig. 1). The overall reaction involves the replacement of an╬▒-proton with an alkyl group. The nucleophilic and electrophilic centers of the enolate ion and methyl iodide are shown (Fig. 2). The enolate ion has its negative charge shared between the oxygen atom and the carbon atom due to resonance, and so both of these atoms are nucleophilic centers. Iodomethane has a polar CŌĆōI bond where the iodine is a weak nucleophilic center and the carbon is a good electrophilic center.
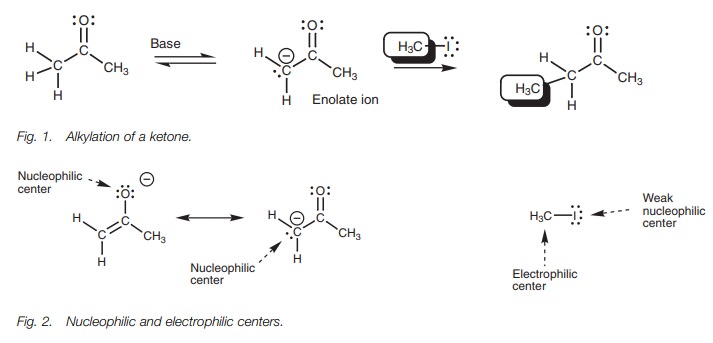
One possible reaction between these molecules
involves the nucleophilic oxy-gen using one of its lone pairs of electrons to
form a new bond to the electrophilic carbon on iodomethane (Fig. 3a). At the same time, the CŌĆōI bond
and both elec-trons move onto iodine to give it a fourth lone pair of electrons
and a negative charge. This reaction is possible, but in practice the product
obtained is more likely to arise from the reaction of the alternative carbanion
structure reacting with methyl iodide (Fig.
3b). This is a more useful reaction since it involves the forma-tion of a
carbonŌĆōcarbon bond and allows the construction of more complex carbon
skeletons.
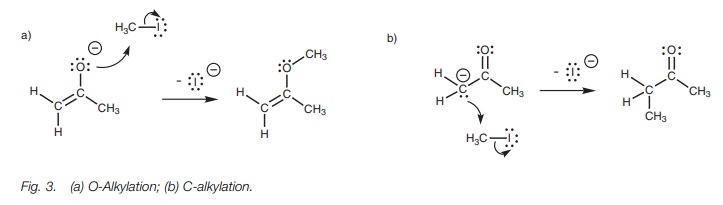
An alternative mechanism to that shown in Fig. 3b, but which gives the same result, starts with the enolate ion. The enolate ion is more stable than the carban-ion since the charge is on the electronegative oxygen and so it is more likely that the reaction mechanism will occur in this manner (Fig. 4). This is a very useful reaction in organic synthesis. However, there are limitations to the type of alkyl halide which can be used in the reaction. The reaction is SN2 with respect to the alkyl halide and so the reaction works best with primary alkyl, primary benzylic, and primary allylic halides. The enolate ion is a strong base and if it is reacted with secondary and tertiary halides, elimination of the alkyl halide takes place to give an alkene.
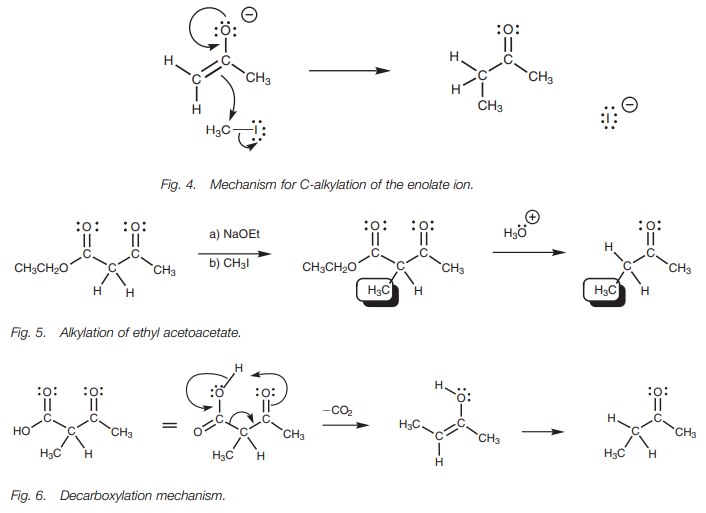
╬▒-Alkylation works well with ketones, but not so well for aldehydes
since thelatter tend to undergo Aldol condensations instead.
The ╬▒-protons of a ketone such as propanone are only
weakly acidic and so a powerful base (e.g. lithium diisopropylamide) is
required to generate the enolate ion required for the alkylation. An
alternative method of preparing the same prod-uct but using a milder base is to
start with ethyl acetoacetate (a ╬▓-keto ester) instead (Fig. 5). The ╬▒-protons in this structure are more acidic
since they are flanked by two carbonyl groups. As a result, the enolate can be
formed using a weaker base such as sodium ethoxide. Once the enolate has been
alky-lated, the ester group can be hydrolyzed and decarboxylated on heating
with aqueous hydrochloric acid. The decarboxylation mechanism involves the ╬▓-keto group and would not occur if this group was absent (Fig. 6). Carbon dioxide is lost and the
enol tautomer is formed. This can then form the keto tautomer by the normal
ketoŌĆōenol tautomerism.
It is possible for two different alkylations to
be carried out on ethyl acetoacetate since there is more than one ╬▒-proton present (Fig. 7).
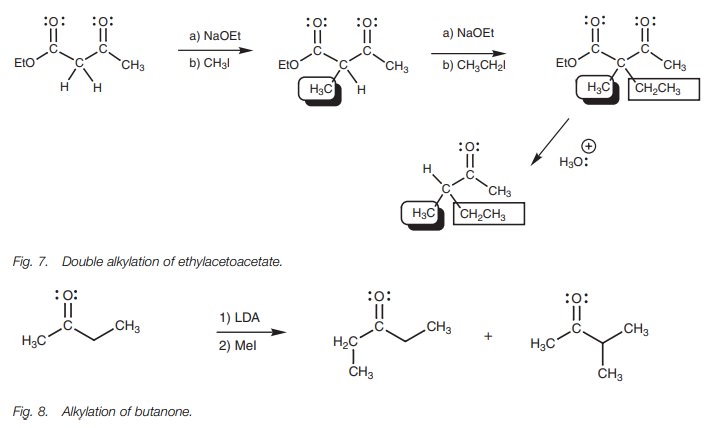
╬▓-keto esters such as ethylacetoacetate are also useful in solving a probleminvolved in the alkylation of unsymmetrical ketones. For example, alkylating butanone with methyl iodide leads to two different products since there are ╬▒-protons on either side of the carbonyl group (Fig. 8). One of these products isobtained specifically by using a ╬▓-keto ester to make the target alkylation site more acidic (Fig. 9).
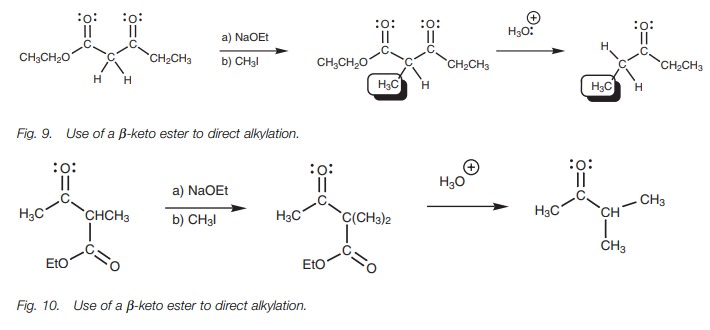
The alternative alkylation product could be obtained by using a different ╬▓-keto ester (Fig. 10).
Aldol reaction
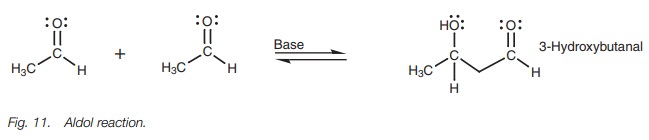
Enolate ions can also react with aldehydes and ketones by nucleophilic addition. The enolate ion acts as the nucleophile while the aldehyde or ketone acts as an electrophile. Since the enolate ion is formed from a carbonyl compound itself, and can then react with a carbonyl compound, it is possible for an aldehyde or ketone to react with itself. We can illustrate this by looking at the reaction of acetaldehyde with sodium hydroxide (Fig. 11). Under these conditions, two molecules of acetaldehyde are linked together to form a ╬▓-hydroxyaldehyde.
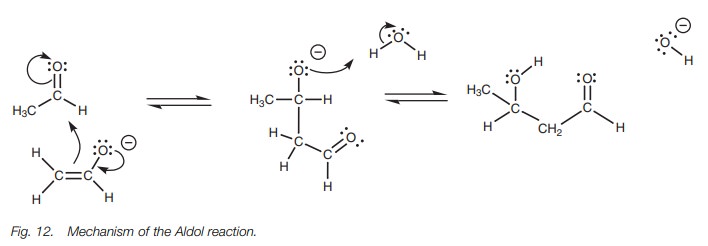
In this reaction, two separate reactions are going on ŌĆō the formation of an enolate ion from one molecule of acetaldehyde,
and the addition of that enolate to a second molecule of acetaldehyde. The
mechanism begins with the formation of the enolate ion. It is important to
realize that not all of the acetaldehyde is converted to the enolate ion and so
we still have molecules of acetaldehyde present in the same solution as the
enolate ions. Since acetaldehyde is susceptible to nucleophilic attack, the
next stage in the mechanism is the nucleo-philic attack of the enolate ion on
acetaldehyde (Fig. 12). The enolate
ion has two nucleophilic centers ŌĆō the carbon and the oxygen ŌĆō but the preferred
reaction is at the carbon atom. The first step is nucleophilic addition of the
aldehyde to form a charged intermediate. The second step involves protonation
of the charged oxygen. Since a dilute solution of sodium hydroxide is used in
this reaction, water is available to supply the necessary proton. (Note that it
would be wrong to show a free proton (H+) since the solution is
alkaline.)
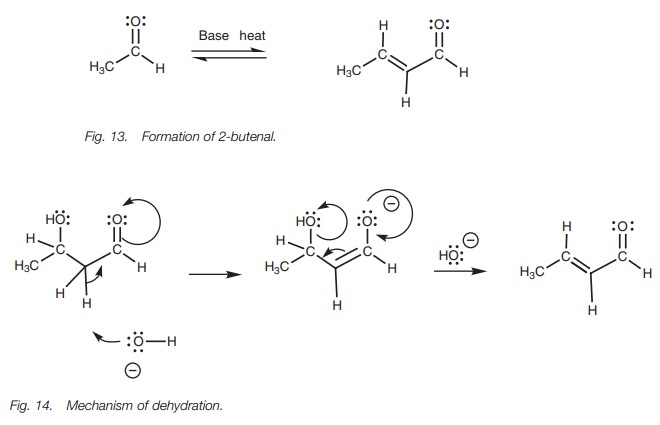
If the above reaction is carried out with heating, then a different product is obtained (Fig. 13). This arises from elimination of a molecule of water from the Aldol reaction product. There are two reasons why this can occur. First of all, the product still has an acidic proton (i.e. there is still a carbonyl group present and an ╬▒-hydrogen next to it). This proton is prone to attack from base. Secondly, thedehydration process results in a conjugated product which results in increased stability. The mechanism of dehydration is shown in Fig. 14. First of all, the acidic proton is removed and a new enolate ion is formed. The electrons in the enolate ion can then move in such a fashion that the hydroxyl group is expelled to give the final product ŌĆō an ╬▒,╬▓-unsaturated aldehyde.
In this example, it is
possi-ble to vary the conditions such that one gets the Aldol reaction product
or the ╬▒,╬▓-unsaturated aldehyde, but in some cases only
the ╬▒,╬▓-unsaturated carbonylproduct is obtained,
especially when extended conjugation is possible. The Aldol reaction is best
carried out with aldehydes. Some ketones will undergo an Aldol reaction, but an
equilibrium is set up between the products and starting materials and it is
necessary to remove the product as it is being formed in order to pull the
reaction through to completion.
Crossed Aldol reaction
So far we have talked about the Aldol reaction
being used to link two molecules of the same aldehyde or ketone, but it is also
possible to link two different carbonyl compounds. This is known as a crossed Aldol reaction. For example,
benzaldehyde and ethanal can be linked in the presence of sodium hydroxide (Fig.15). In this example, ethanal reacts
with sodium hydroxide to form the enolate ionwhich then reacts with benzaldehyde.
Elimination of water occurs easily to give an extended conjugated system
involving the aromatic ring, the double bond, and the carbonyl group.
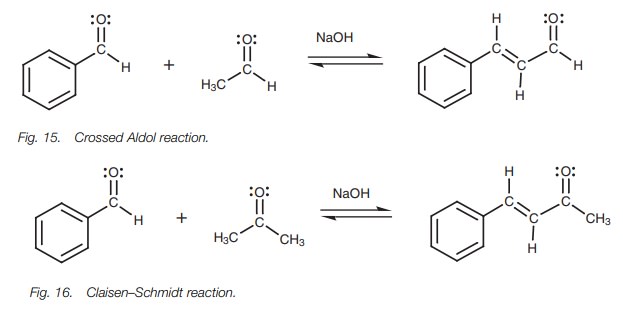
This reaction works well because the
benzaldehyde has no ╬▒-protons and cannot form an enolate ion.
Therefore, there is no chance of benzaldehyde under-going self-condensation. It
can only act as the electrophile for another enolate ion. However, what is to
stop the ethanal undergoing an aldol addition with itself as previously
described (Fig. 11)?
This reaction can be limited by only having benzaldehyde and sodium hydrox-ide initially present in the reaction flask. Since benzaldehyde has no ╬▒-protons, no reaction can take place. A small quantity of ethanal can now be added. Reaction with excess sodium hydroxide turns most of the ethanal into its enolate ion. There will only be a very small amount of ŌĆśfreeŌĆÖ ethanal left compared to benzaldehyde and so the enolate ion is more likely to react with benzaldehyde. Once the reaction is judged to have taken place, the next small addition of ethanal can take place and the process is repeated.
Ketones and aldehydes can also be linked by the
same method ŌĆō a reaction known as the ClaisenŌĆōSchmidt
reaction. The most successful reactions are those where the aldehyde does not
have an╬▒-proton (Fig.
16).
Related Topics