Chapter: Organic Chemistry: Carboxylic acids and carboxylic acid derivatives
Reactions of Carboxylic acids and carboxylic acid derivatives
REACTIONS
Key Notes
Acid–base reactions
Carboxylic
acids form water soluble carboxylate salts when treated with a base.
Interconversion of acid derivatives
Reactive
acid derivatives can be converted to less reactive acid derivatives. Acid
chlorides can be converted to acid anhydrides, esters, or amides; acid
anhydrides can be converted to esters or amides; and esters can be con-verted
to amides. Transesterification is also possible by dissolving an ester into an
excess of alcohol in the presence of an acid catalyst.
Hydrolysis
Acid
chlorides and acid anhydrides are sufficiently reactive to be hydrolyzed by
water to their constituent carboxylic acids. Heating under basic or acidic
conditions is preferable for the hydrolysis of less reactive esters and amides.
The reaction is another example of nucleophilic substi-tution. Under neutral or
acidic conditions, the nucleophile is water. Under basic conditions, the
nucleophile is the hydroxide ion and the reaction is driven by the irreversible
formation of the carboxylate ion. Amides can also be effectively hydrolyzed
under acid conditions due to the formation of an ammonium salt. In contrast,
the acid-catalyzed hydrolysis of esters is an equilibrium reaction.
Friedel–Crafts acylation
Aromatic
rings can be treated with acid chlorides in the presence of a Lewis acid to
give an aromatic ketone.
Grignard reaction
Acid
chlorides and esters react twice with Grignard reagents to form ter-tiary
alcohols, with the introduction of two alkyl substituents. Carboxylic acids and
Grignard reagents react together in an acid–base reaction which serves no
synthetic value.
Organolithium reactions
Esters
react with organolithium reagents to produce tertiary alcohols in a similar
process to that described for Grignard reagents. Carboxylic acids have to be
protected to prevent destruction of the organolithium reagent in an acid–base
reaction.
Organocuprate reactions
Acid chlorides
can be treated with an organocuprate reagent
to give ketones. The reaction mechanism is radical based and is not a
nucleophilic substitution.
Reduction
Lithium
aluminum hydride (LiAlH4) is used to convert carboxylic acids, acid
chlorides, acid anhydrides, and esters to primary alcohols. Amides are reduced
to amines. Hindered hydride reagents are less reactive and can be used to
convert acid chlorides or esters to aldehydes. Borane can be used to reduce
carboxylic acids to primary alcohols when nitro groups are present.
Sodium
borohydride does not reduce carboxylic acids or their derivatives and can be
used to reduce aldehydes and ketones without affecting car-boxylic acids or
acid derivatives.
Dehydration of primary amides
Primary
amides are dehydrated to nitriles on treatment with a dehydrating agent such as
thionyl chloride.
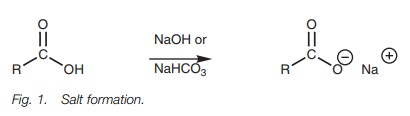
Acid–base reactions
Since carboxylic acids have an acidic proton
(CO2H), they form water soluble carboxylate salts on
treatment with a base (e.g. sodium hydroxide or sodium bicarbonate).
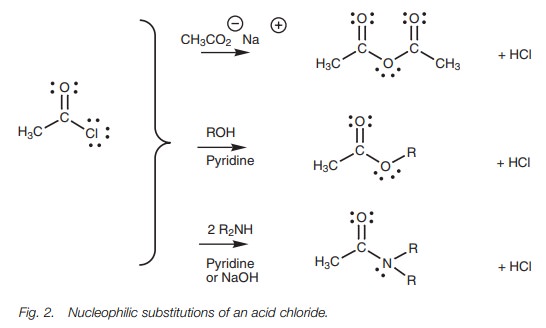
Interconversion of acid derivatives
Reactive acid derivatives can be converted to less
reactive acid derivatives by nucleophilic substitution. This means that acid
chlorides can be converted to acid anhydrides, esters, and amides (Fig. 2). Hydrochloric acid is released
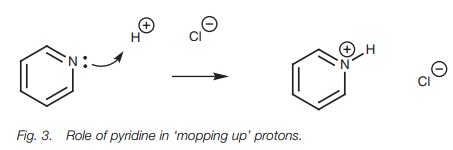
in these reactions and this may lead to side reactions. As a result, pyridine
or sodium hydroxide may be added in order to mop up the hydrochloric acid (Fig. 3).
Acid anhydrides can be converted to esters and amides but not to acid chlorides (Fig. 4).
Esters can be converted to amides but not to acid chlorides or acid anhydrides (Fig. 5).
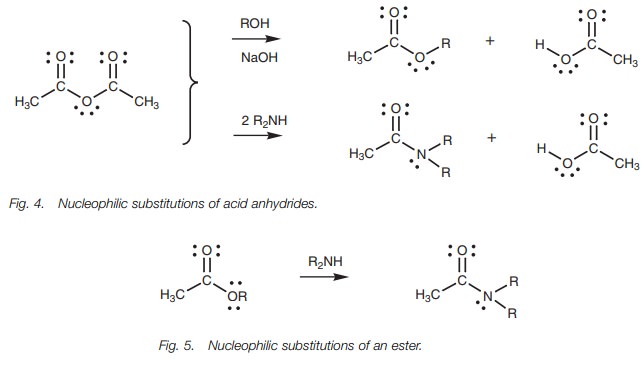
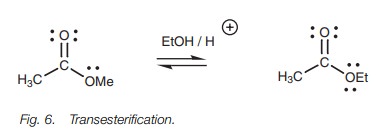
Esters can also be converted by nucleophilic
substitution from one type of ester to another – a process called transesterification. For example, a
methyl ester could be dissolved in ethanol in the presence of an acid catalyst
and converted to an ethyl ester (Fig. 6).
The reaction is an equilibrium reaction, but if the alcohol to be introduced is
used as solvent, it is in large excess and the equilibrium is shifted to the
desired ester. Furthermore, if the alcohol to be replaced has a low boiling
point, it can be distilled from the reaction as it is substituted, thus
shifting the equilibrium to the desired product.
Amides are the least reactive of the acid
derivatives and cannot be converted to acid chlorides, acid anhydrides, or
esters.
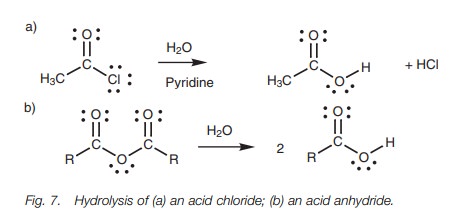
Hydrolysis
Reactive acid derivatives (i.e. acid chlorides
and acid anhydrides) are hydrolysed by water to give the constituent carboxylic
acids (Fig. 7). The reaction is another example
of nucleophilic substitution
where water acts
as the nucleophile. Hydrochloric acid is a byproduct
from the hydrolysis of an acid chloride, so pyridine is often added to the
reaction mixture to mop it up (Fig. 3).
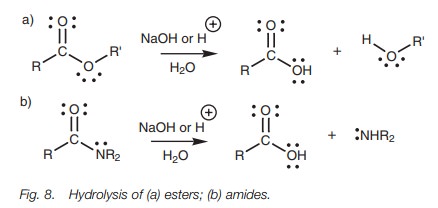
Esters and amides are less reactive and so the
hydrolysis requires more forcing conditions using aqueous sodium hydroxide or
aqueous acid with heating (Fig. 8).
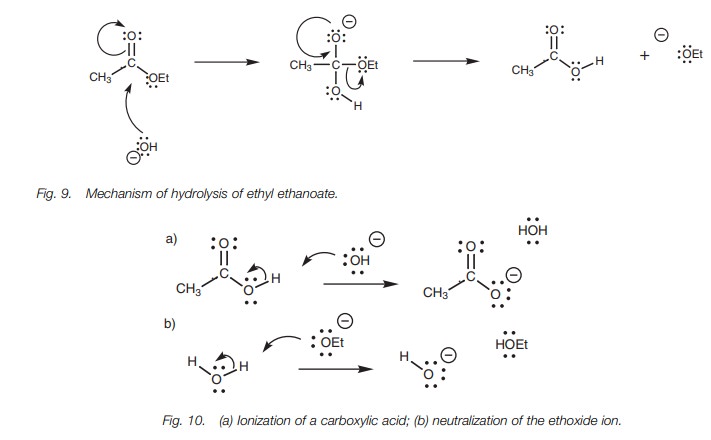
Under basic conditions, the hydroxide ion acts
as the nucleophile by the normal mechanism for nucleophilic substitution. For
example, the mechanism of hydrolysis of ethyl acetate is as shown (Fig. 9). However, the mechanism does not
stop here. The carboxylic acid which is formed reacts with sodium hydroxide to
form a water soluble carboxylate ion (Fig.
10a). Furthermore, the ethoxide ion which is lost from the molecule is a
stronger base than water and undergoes proto-nation (Fig. 10b). The basic hydrolysis of an ester is also known as saponification and produces a water
soluble carboxylate ion.
The same mechanism is involved in the basic
hydrolysis of an amide and also results in a water soluble carboxylate ion. The
leaving group from an amide is initially charged (i.e. R2N: ).
However, this is a strong base and reacts with water to form a free amine plus
a hydroxide ion.
In the basic hydrolysis of esters and amides,
the formation of a carboxylate ion is irreversible and so serves to drive the
reaction to completion.
In order to isolate the carboxylic acid rather than the salt, it is necessary to add acid (e.g. dilute HCl) to the aqueous solution. The acid protonates the carboxylate salt to give the carboxylic acid which (in most cases) is no longer soluble in aqueous solution and precipitates out as a solid or as an oil.
The mechanism for acid–catalyzed hydrolysis (Fig. 11) involves water acting as a
nucleophile. However, water is a poor nucleophile since it gains an unfavorable
positive charge when it forms a bond. Therefore, the carbonyl group has to be
activated which occurs when the carbonyl oxygen is protonated by the acid
catalyst (Step 1). Nucleophilic attack by water is now favored since it
neutralizes the unfavorable positive charge on the carbonyl oxygen (Step 2).
The intermediate has a positive charge on the oxygen derived from water, but
this is neutralized by losing the attached proton such that the oxygen gains
the electrons in the O–H bond (Step 3). Another protonation now takes place
(Step 4). This is necessary in order to convert a poor leaving group (the
methoxide ion) into a good leaving group (methanol). The π bond can now be reformed (Step 5) with loss of methanol. Finally,
water can act as a base to remove the proton from the carbonyl oxygen (Step 6).
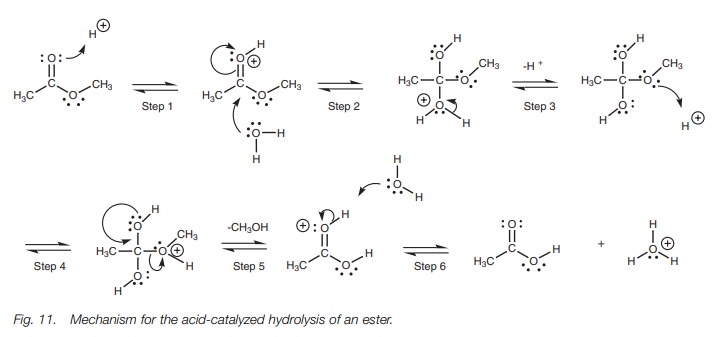
The acid-catalyzed hydrolysis of an ester is
not as effective as basic hydrolysis since all the steps in the mechanism are
reversible and there is no salt formation to pull the reaction through to
products. Therefore, it is important to use an excess of water in order to
shift the equilibria to the products. In contrast to esters, the hydrolysis of
an amide in acid does result in the formation of an ion (Fig. 12). The leaving group here is an amine and since amines are
basic, they will react with the acid to form a water soluble aminum ion. This
is an irreversible step which pulls the equilibrium through to the products.
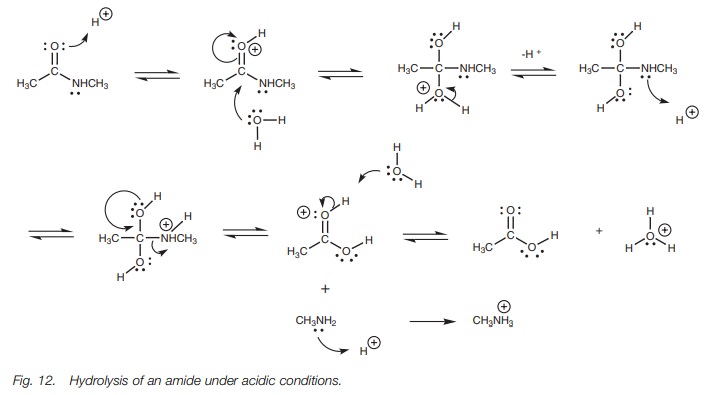
In the acid-catalyzed hydrolysis of an ester,
only a catalytic amount of acid is required since the protons used during the
reaction mechanism are regenerated. However with an amide, at least one
equivalent of acid is required due to the ionization of the amine.
Friedel–Crafts acylation
Acid chlorides can react with aromatic rings in
the presence of a Lewis acid to give aromatic ketones (Fig. 13). The reaction involves formation of an acylium ion from
the acid chloride, followed by electrophilic substitution of the aromatic ring
.
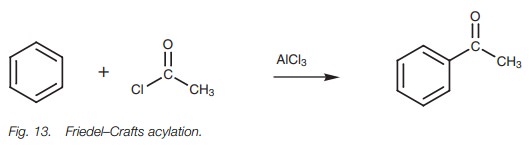
Grignard reaction
Acid chlorides and esters react with two
equivalents of a Grignard reagent to
produce a tertiary alcohol where two extra alkyl groups are provided by the
Grignard reagent (Fig. 14).
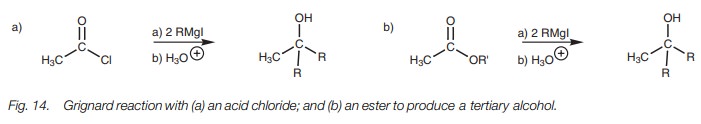
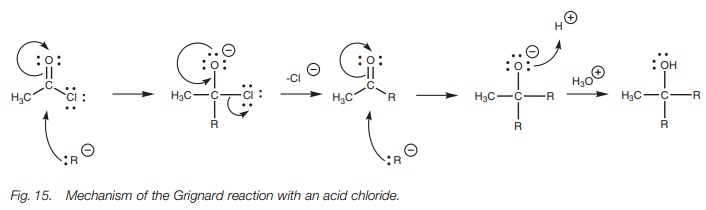
There are two reactions involved in this
process (Fig. 15). The acid chloride
reacts with the first equivalent of Grignard reagent in a typical nucleophilic
substitution to produce an intermediate ketone. However, this ketone is also
reactive to Grignard reagents and immediately reacts with a second equivalent
of Grignard reagent by the nucleophilic addition mechanism described for
aldehydes and ketones.
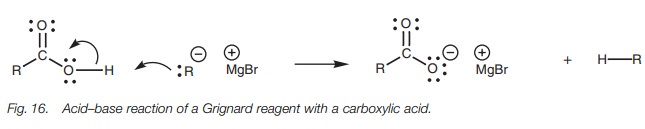
Carboxylic acids react with Grignard reagents
in an acid–base reaction resulting in formation of the carboxylate ion and
formation of an alkane from the Grignard reagent (Fig. 16). This has no synthetic use and it is important to protect
carboxylic acids when carrying out Grignard reactions on another part of the
molecule so that the Grignard reagent is not wasted.
Carboxylic acids react with Grignard reagents
in an acid–base reaction resulting in formation of the carboxylate ion and
formation of an alkane from the Grignard reagent (Fig. 16). This has no synthetic use and it is important to protect
carboxylic acids when carrying out Grignard reactions on another part of the
molecule so that the Grignard reagent is not wasted.
Organolithium reactions
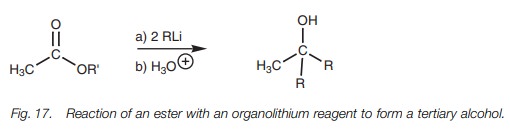
Esters react with two equivalents of an
organolithium reagent to give a tertiary alcohol where two of the alkyl groups
are derived from the organolithium reagent (Fig.
17). The mechanism of the reaction is the same as that described in the
Grignard reaction, that is, nucleophilic substitution to form a ketone followed
by nucleophilic addition. It is necessary to protect any carboxylic acids present
when carrying out organolithium reactions since one equivalent of the
organo-lithium reagent would be wasted in an acid–base reaction with the
carboxylic acid.
Organocuprate reactions
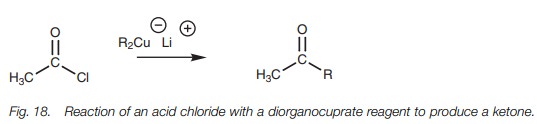
Acid chlorides react with diorganocuprate
reagents to form ketones (Fig. 18).
Like the Grignard reaction, an alkyl group displaces the chloride ion to
produce a ketone. However, unlike the Grignard reaction, the reaction stops at
the ketone stage. The mechanism is thought to be radical based rather than a
nucle-ophilic substitution. This reaction does not take place with carboxylic
acids, acid anhydrides, esters, or amides.
Reduction
Carboxylic acids, acid chlorides, acid
anhydrides and esters are reduced to primary alcohols on treatment with lithium
aluminum hydride (LiAlH4 ;Fig.
19). The reaction involves nucleophilic substitution by a hydride ion to
give an intermediate aldehyde. This cannot be isolated since the aldehyde
immediately undergoes a nucleophilic addition reaction with another hydride
ion. The detailed mechanism is as shown in
Fig. 21.
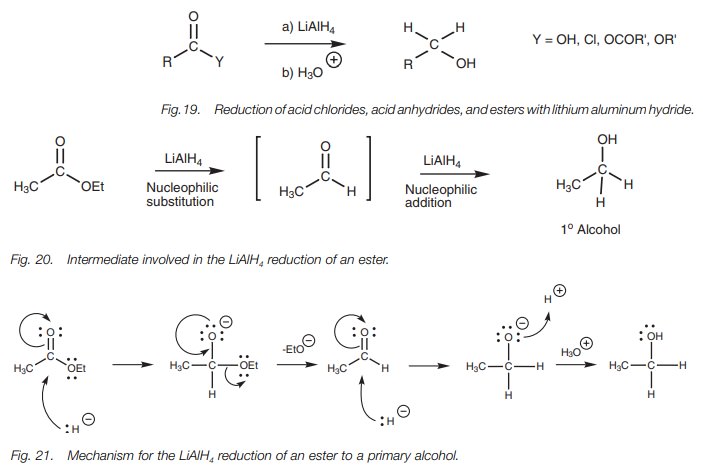
Amides differ from carboxylic acids and other
acid derivatives in their reaction with LiAlH4 . Instead of forming
primary alcohols, amides are reduced to amines (Fig. 22). The mechanism (Fig.
23) involves addition of the hydride ion to form an intermediate which is
converted to an organoaluminum intermediate. The differ-ence in this mechanism
is the intervention of the nitrogen’s lone pair of electrons. These are fed
into the electrophilic center to eliminate the oxygen which is then followed by
the second hydride addition.
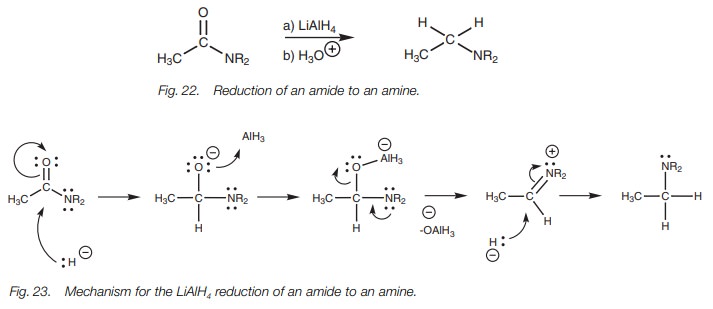
Although acid chlorides and acid anhydrides are
converted to tertiary alco-hols with LiAlH4, there is little
synthetic advantage in this since the same reac-tion can be achieved on the
more readily available esters and carboxylic acids. However, since acid
chlorides are more reactive than carboxylic acids, they can be treated with a
milder hydride-reducing agent and this allows the synthesis of aldehydes (Fig. 24). The hydride reagent used (lithium
tri-tert-butoxyaluminum hydride)
contains three bulky alkoxy groups which lowers the reactivity of the remaining
hydride ion. This means that the reaction stops after nucleophilic substitution
with one hydride ion. Another sterically hindered hydride reagent –
diisobutylaluminum hydride (DIBAH) – is used to reduce esters to aldehydes (Fig. 24). Normally low temperatures are
needed to avoid over-reduction.
Borane (B2H6) can be used
as a reducing agent to convert carboxylic acids to pri-mary alcohols. One
advantage of using borane rather than LiAlH4 is the fact that the
former does not reduce any nitro groups which might be present. LiAlH4
reduces a nitro group (NO2) to an amino group (NH2).
It is worth noting that carboxylic acids and
acid derivatives are not reduced by the milder reducing agent – sodium
borohydride (NaBH4). This reagent can there-fore be used to reduce
aldehydes and ketones without affecting any carboxylic acids or acid
derivatives which might be present.
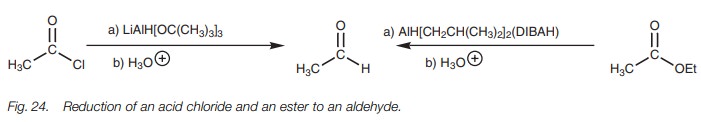
Dehydration
Primary amides are dehydrated to nitriles using
a dehydrating agent such as of primary amides thionyl chloride (SOCl2), phosphorus
pentoxide (P2O5), phosphoryl trichloride (POCl3),
or acetic anhydride (Fig. 25).
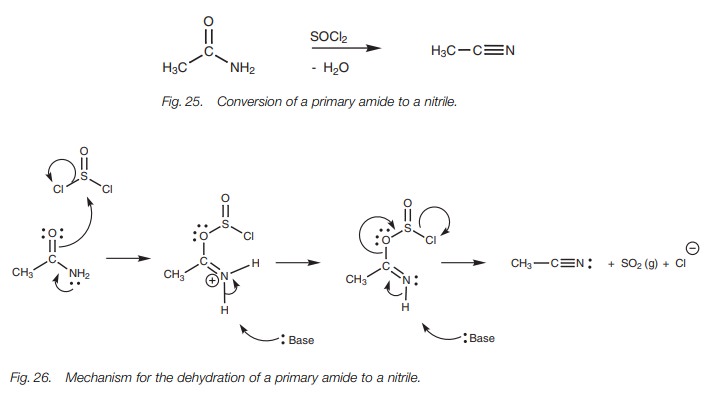
The mechanism for the dehydration of an amide
with thionyl chloride is shown in Fig. 26.
Although the reaction is the equivalent of a dehydration, the mechanism shows
that water itself is not eliminated. The reaction is driven by the loss of
sulfur dioxide as a gas.
Related Topics