Chapter: Organic Chemistry: Carboxylic acids and carboxylic acid derivatives
Preparations of carboxylic acid derivatives
PREPARATIONS OF CARBOXYLIC ACID DERIVATIVES
Key Notes
Acid chlorides
Acid
chlorides are synthesized by treating carboxylic acids with thionyl chloride,
phosphorus trichloride, or oxalyl chloride. The carboxylic acid reacts with
the reagent to
release a chloride
ion which then
acts as a nucleophile with the reaction intermediate
to form the acid chloride.
Acid anhydrides
Acid
anhydrides are best prepared by treating acid chlorides with a car- boxylate
salt. Cyclic anhydrides can be synthesized from acyclic di-acids by heating.
Esters
Esters
are prepared by the nucleophilic substitution of acid chlorides or acid
anhydrides with alcohols, the nucleophilic substitution of carboxylic acids
with alcohol in the presence of a catalytic amount of mineral acid, the SN2
nucleophilic substitution of an alkyl halide with a carboxylate ion, and
finally the reaction of carboxylic acids with diazomethane to give methyl
esters.
Amides
Acid
chlorides can be converted to primary, secondary, and tertiary amides by
reaction with ammonia, primary amines, and secondary amines, respec-
tively. Acetic anhydride
can be treated
with amines to
synthesize ethanamides. Carboxylic acids and amines react together to
form a salt. Some salts can be converted to amides by strong heating to remove
water.
Acid chlorides
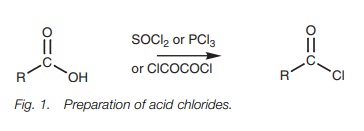
Acid chlorides can be prepared from carboxylic
acids using thionyl chloride (SOCl2), phosphorus trichloride (PCl3),
or oxalyl chloride (ClCOCOCl Fig. 1).
The mechanism for these reactions is quite involved, but in general involves the OH group of the carboxylic acid acting as a nucleophile to form a bond to the reagent and displacing a chloride ion. This has three important consequences. First of all, the chloride ion can attack the carbonyl group to introduce the required chlorine atom. Secondly, the acidic proton is no longer present and so an acid–base reaction is prevented. Thirdly, the original OH group is converted into a good leaving group and is easily displaced once the chloride ion makes its attack. The reaction of a carboxylic acid with thionyl chloride follows the general pathway shown in Fig. 2.
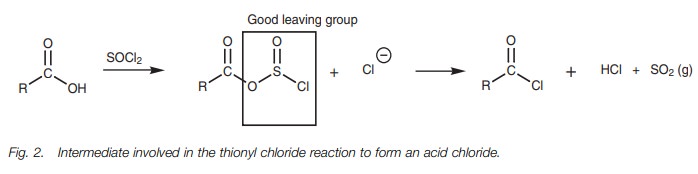
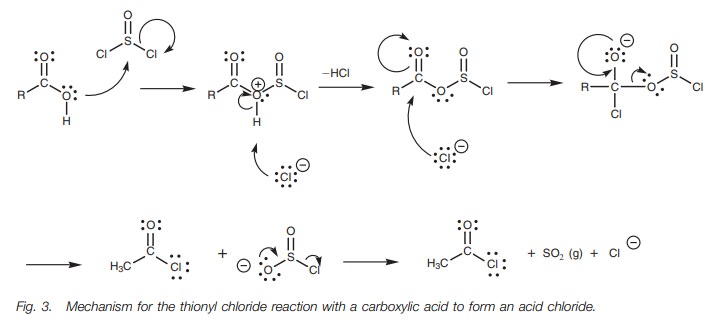
The leaving group (SO2Cl)
spontaneously fragments to produce hydrochloric acid and sulfur dioxide. The
latter is lost as a gas which helps to drive the reaction to completion. The
detailed mechanism is shown in Fig. 3.
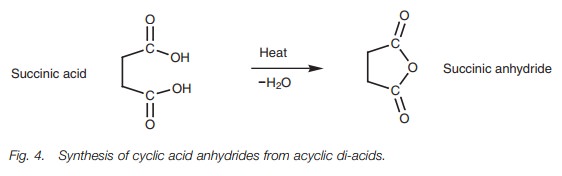
Acid anhydrides
Acid anhydrides are best prepared by treating
acid chlorides with a carboxylate salt . Carboxylic acids are not easily
converted to acid anhydrides directly. However five-membered and six-membered
cyclic anhydrides can be synthesized from diacids by heating the acyclic
structures to eliminate water (Fig. 4).
Esters
There are many different ways in which esters
can be synthesized. A very effective method is to react an acid chloride with
an alcohol in the presence of pyridine . Acid anhydrides also react with
alcohols to give esters, but are less reactive . Furthermore, the reaction is
wasteful since half of the acyl content in the acid anhydride is wasted as the
leaving group (i.e. the carboxylateion). This is not a problem if the acid
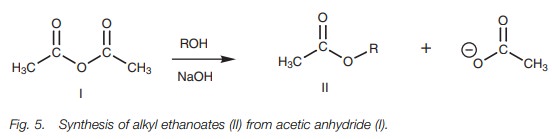
anhydride is cheap and readily available. For example, acetic anhydride is
useful for the synthesis of a range of acetate esters (Fig. 5).
A very common method of synthesizing simple
esters is to treat a carboxylic acid with a simple alcohol in the presence of a
catalytic amount of mineral acid (Fig. 6).
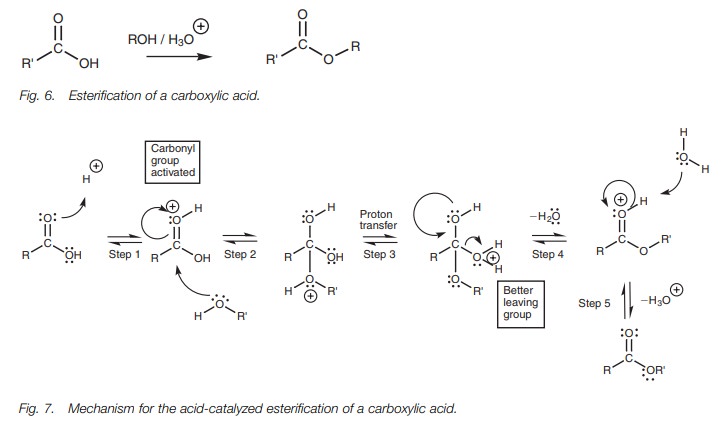
The acid catalyst is required since there are two difficult steps in the
reac-tion mechanism. First of all, the alcohol molecule is not a good
nucleophile and so the carbonyl group has to be activated. Secondly, the OH group
of the carboxylic acid is not a good leaving group and this has to be converted
into a better leaving group. The mechanism (Fig.
7) is another example of nucleophilic substitution. In the first step, the
carbonyl oxygen forms a bond to the acidic proton. This results in the carbonyl
oxygen gaining a positive charge. This makes the carbonyl carbon more
electrophilic and activates it to react with the weakly nucleophilic alcohol.
In the second step, the alcohol uses a lone pair of electrons to form a bond to
the carbonyl carbon. At the same time, the carbonyl π bond breaks and both electrons move onto the carbonyl oxygen to
form a lone pair of electrons and thus neutral-ize the positive charge.
Activation of the carbonyl group is important since the incoming alcohol gains
an unfavorable positive charge during this step. In the third stage, a proton
is transferred from the original alcohol portion to the OH group which we want
to remove. By doing so, the latter moiety becomes a much better leaving group.
Instead of a hydroxide ion, we can now remove a neutral water molecule. This is
achieved in the fourth step where the carbonyl π bond is reformed and the water molecule is expelled.
All the steps in the reaction mechanism are in
equilibrium and so it is important to use the alcohol in large excess (i.e. as
solvent) in order to drive the equilibrium to products. This is only practical
with cheap and readily available alcohols such as methanol and ethanol. On the
other hand, if the carboxylic acid is cheap and readily available it could be
used in large excess instead.
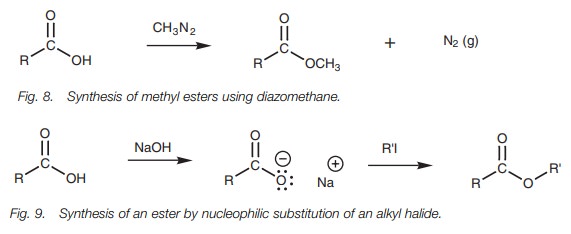
An excellent method of preparing methyl esters is to treat carboxylic acids with diazomethane (Fig. 8). Good yields are obtained because nitrogen is formed as one of the products and since it is lost from the reaction mixture, the reaction is driven to completion. However, diazomethane is an extremely hazardous chemical which can explode, and strict precautions are necessary when using it.
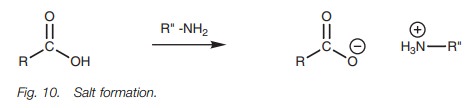
Lastly, the carboxylic acid can be converted to
a carboxylate ion and then treated with an alkyl halide (Fig. 9). The reaction involves the SN2 nucleophilic
substitution of an alkyl halide and so the reaction works best with primary
alkyl halides.
Amides
Amides can be prepared from acid chlorides by
nucleophilic substitution . Treatment with ammonia gives a primary amide,
treatment with a primary amine gives a secondary amide, and treatment with a
secondary amine gives a tertiary amide. Tertiary amines cannot be used in this
reaction because they do not give a stable product.
Two equivalents of amine are required for the
above reactions since one equiv- alent of the amine is used up in forming a
salt with the hydrochloric acid which is produced in the reaction. This is
clearly wasteful on the amine, especially if the amine is valuable. To avoid
this, one equivalent of sodium hydroxide can be added to the reaction in order
to neutralize the HCl.
Amides can also be synthesized from acid
anhydrides and esters , but in general these reactions offer no advantage over
acid chlorides since acid anhydrides and esters are less reactive. Furthermore,
with acid anhydrides, half of the parent carboxylic acid is lost as the leaving
group. This is wasteful and so acid anhydrides are only used for the synthesis
of amides if the acid anhydride is cheap and freely available (e.g. acetic
anhydride).
The synthesis of amides directly from carboxylic acids is not easy since the reac-tion of an amine with a carboxylic acid is a typical acid–base reaction resulting in the formation of a salt (Fig. 10). Some salts can be converted to an amide by heat-ing strongly to expel water, but there are better methods available as previously described.
Related Topics