Chapter: Organic Chemistry: Carboxylic acids and carboxylic acid derivatives
Reactivity of Carboxylic acids and carboxylic acid derivatives
REACTIVITY
Key Notes
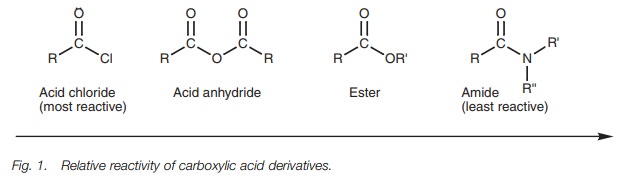
Reactivity order
Acid
chlorides are more reactive than acid anhydrides towards nucleophilic
substitution. Acid anhydrides are more reactive than esters, and esters are
more reactive than amides. It is possible to convert a reactive acid
deriva-tive to a less reactive acid derivative, but not the other way round.
Electronic factors
The
relative reactivity of the four different acid derivatives is determined by the
relative electrophilicities of the carbonyl carbon atom. Neighboring
electronegative atoms increase the electrophilicity of the carbonyl group
through an inductive effect. The greater the electronegativity of the
neigh-boring atom, the greater the effect. Chlorine is more electronegative
than oxygen, and oxygen is more electronegative than nitrogen. Thus, acid
chlo-rides are more reactive than acid anhydrides and esters, while amides are
the least reactive of the acid derivatives. Resonance effects play a role in
diminishing the electrophilic character of the carbonyl carbon. Neighboring
atoms containing a lone pair of electrons can feed these electrons into the
carbonyl center to form a resonance structure where the carbonyl π bond is broken. This
resonance is significant in amines where nitrogen is a good nucleophile, but is
insignificant in acid chlorides where chlorine is a poor nucleophile. Resonance
involving oxygen is weak but significant enough to explain the difference in
reactivity between acid anhydrides and esters. Since the resonance in acid
anhydrides is split between two carbonyl groups, the decrease in reactivity is
less significant than in esters.
Steric factors
Bulky
groups attached to the carbonyl group can hinder the approach of nucleophiles
and result in lowered reactivity. Bulky nucleophiles will also react more
slowly.
Carboxylic acids
Carboxylic
acids are more likely to undergo acid–base reactions with nucleophiles rather
than nucleophilic substitution. Nucleophilic substitu-tion requires prior
activation of the carboxylic acid.
Reactivity order
Acid chlorides can be converted to acid
anhydrides, esters, or amides. These reactions are possible because acid
chlorides are the most reactive of the four carboxylic acid derivatives.
Nucleophilic substitutions of the other acid derivatives are more limited
because they are less reactive. For example, acid anhydrides can be used to
synthesize esters and amides, but cannot be used to synthesize acid chlorides.
The possible nucleophilic reactions for each carboxylic acid derivative depends
on its reactivity with respect to the other acid derivatives (Fig. 1). Reactive acid derivatives can
be converted to less reactive (more stable) acid derivatives, but not the other
way round. For example, an ester can be converted to an amide, but not to an
acid anhydride.
Electronic factors
But why is there this difference in reactivity?
The first step in the nucleophilic substitution mechanism (involving the
addition of a nucleophile to the electrophilic carbonyl carbon) is the
rate-determining step. Therefore, the more electrophilic this carbon is, the
more reactive it will be. The nature of Y has a significant effect in this
respect (Fig. 2).

Y is linked to the acyl group by an
electronegative heteroatom (Cl, O, or N) which makes the carbonyl carbon more
electrophilic. The extent to which this happens depends on the
electronegativity of Y. If Y is strongly electronegative (e.g. chlorine), it
has a strong electron-withdrawing effect on the carbonyl carbon making it more
electrophilic and more reactive to nucleophiles. Since chlorine is more
electronegative than oxygen, and oxygen is more electronegative than nitrogen,
acid chlorides are more reactive than acid anhydrides and esters, while acid
anhydrides and esters are more reactive than amides.
The electron-withdrawing effect of Y on the
carbonyl carbon is an inductive effect. With amides, there is an important
resonance contribution which decreases
the electrophilicity of the carbonyl carbon (Fig. 3). The nitrogen has a lone pair of electrons which can form a
bond to the neighboring carbonyl carbon. As this new bond is formed, the weak π bond breaks and both electrons move onto oxygen to give it a third
lone pair of electrons and a negative charge. Since the nitrogen’s lone pair of
electrons is being fed into the carbonyl group, the carbonyl carbon becomes
less electrophilic and is less prone to attack by an incoming nucleophile.
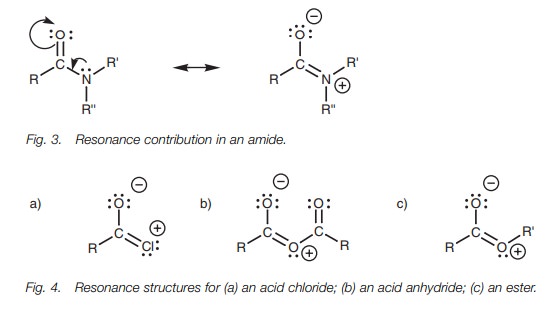
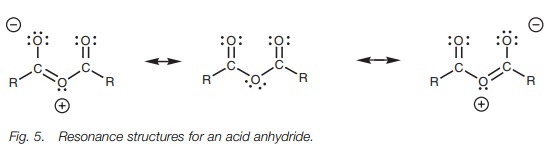
In theory, this resonance could also occur in acid chlorides, acid anhydrides, and esters to give resonance structures (Fig. 4). However, the process is much less important since oxygen and chlorine are less nucleophilic than nitrogen. In these structures, the positive charge ends up on an oxygen or a chlorine atom. These atoms are more electronegative than nitrogen and less able to stabilize a positive charge. These resonance structures might occur to a small extent with esters and acid anhydrides, but are far less likely in acid chlorides. This trend also matches the trend in reactivity.
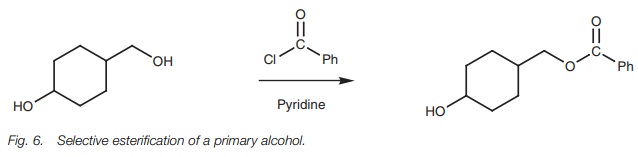
Although the resonance effect is weak in esters
and acid anhydrides, it can explain why acid anhydrides are more reactive than
esters. Acid anhydrides have two carbonyl groups and so resonance can take
place with either carbonyl group (Fig. 5).
As a result, the lone pair of the central oxygen is ‘split’ between both groups
which means that the resonance effect is split between both carbonyl groups.
This means that the effect of resonance at any one carbonyl group is diminished
and it will remain strongly electrophilic. With an ester, there is only one
carbonyl group and so it experiences the full impact of the resonance effect.
Therefore, its electrophilic strength will be diminished relative to an acid
anhydride.
Steric factors
Steric factors can play a part in the reactivity of acid derivatives. For example, a bulky group attached to the carbonyl group can hinder the approach of nucleophiles and hence lower reactivity. The steric bulk of the nucleophile can also have an influence in slowing down the reaction. For example, acid chlorides react faster with primary alcohols than they do with secondary or tertiary alcohols. This allows selective esterification if a molecule has more than one alcohol group present (Fig. 6).
Carboxylic acids
Where do carboxylic acids fit into the
reactivity order described above? The nucleophilic substitution of carboxylic
acids is complicated by the fact that an acidic proton is present. Since most
nucleophiles can act as bases, the reaction of a carboxylic acid with a
nucleophile results in an acid–base reaction rather than nucleophilic
substitution.
However, carboxylic acids can undergo
nucleophilic substitution if they are activated in advance.
Related Topics