Chapter: Organic Chemistry: Carboxylic acids and carboxylic acid derivatives
Preparations of carboxylic acids
PREPARATIONS OF CARBOXYLIC ACIDS
Key Notes
Functional group transformations
Primary
alcohols and aldehydes are converted to carboxylic acids by oxida-tion. Acid
chlorides, acid anhydrides, esters, and amides can be hydrolyzed to their
parent carboxylic acids, but only the hydrolysis of esters serves a useful
synthetic role if the ester is being used as a protecting group.
C–C bond formation
Aromatic
carboxylic acids are obtained by the oxidation of alkyl benzenes.
Alkyl
halides can be converted to carboxylic acids where the carbon chain has been
extended by one carbon unit. Two methods are possible. The alkyl halide can be
converted to a cyanide which is then hydrolyzed. Alterna- tively, the alkyl
halide can be converted to a Grignard reagent then treated with carbon dioxide.
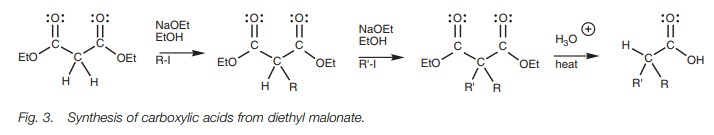
A range of carboxylic acids can be prepared by alkyl- ating diethyl malonate,
then hydrolyzing and decarboxylating the product.
Bond cleavage
Alkenes can
be cleaved across
the double bond
by potassium perman- ganate. Carboxylic acids are formed
if a vinylic proton is present.
Functional group transformations
Carboxylic acids can be obtained by the
oxidation of primary alcohols or aldehydes, the hydrolysis of nitriles , or the
hydrolysis of esters which
can be used
as protecting groups
for carboxylic acids. Amides
can also be
hydrolyzed to carboxylic
acids. However, fiercer reaction conditions are required due
to the lower reactivity of amides and so amides are less useful as carboxylic
acid protecting groups.
Although acid chlorides and anhydrides are
easily hydrolyzed to carboxylic acids,
the reaction serves
no synthetic purpose
since acid chlorides
and acid anhydrides are
synthesized from carboxylic acids in the first place. They are also too
reactive to be used as protecting groups.
C–C bond formation
Aromatic carboxylic acids can be obtained by
oxidation of alkyl benzenes. It does not matter how large the alkyl group is,
since they are all oxidized to a benzoic acid structure.
There are two methods by which alkyl halides
can be converted to a carboxylic acid and in both cases, the carbon chain is
extended by one carbon. One method involves substituting the halogen with a
cyanide ion , then hydrolysing the cyanide group. This works best with primary
alkyl halides.
The other method involves the formation of a
Grignard reagent which is then treated with carbon dioxide (Fig. 1b).
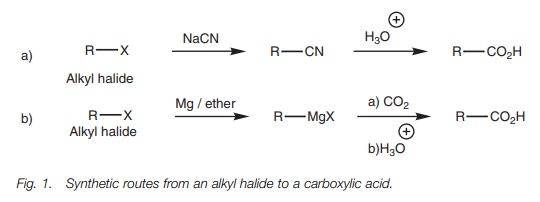
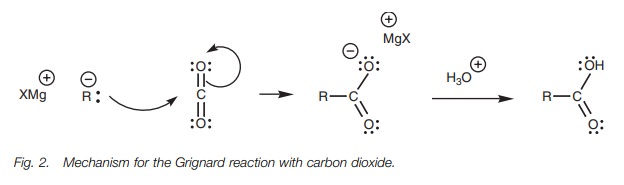
The mechanism for the Grignard reaction is
similar to the nucleophilic addition of a Grignard reagent to an aldehyde or
ketone.
A range of carboxylic acids can be prepared by alkylating diethyl malonate, then hydrolyzing and decarboxylating the product.
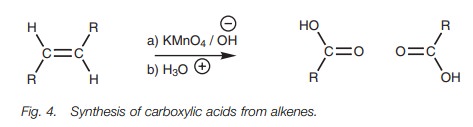
Bond cleavage
Alkenes can be cleaved with potassium
permanganate to produce carboxylic acids (Fig.
4). A vinylic proton has to be present, that is a proton directly attached
to the double bond.
Related Topics