Environmental Chemistry - Stratospheric pollution | 11th Chemistry : UNIT 15 : Environmental Chemistry
Chapter: 11th Chemistry : UNIT 15 : Environmental Chemistry
Stratospheric pollution
Stratospheric
pollution
At
high altitudes to the atmosphere consists of a layer of ozone (O3)
which acts as an umbrella or shield for harmful UV radiations. It protects us
from harmful effect such as skin cancer. UV radiation can convert molecular
oxygen into ozone as shown in the following reaction.
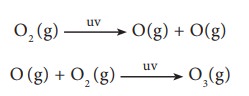
Ozone
gas is thermodynamically unstable and readily decomposes to molecular oxygen.
Depletion of Ozone Layer (Ozone hole)
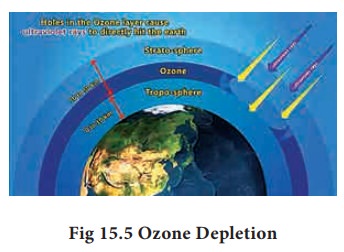
In
recent years, a gradual depletion of this protective ozone layer has been
reported. Nitric oxide and CFC are found to be most responsible for depletion
of ozone layer.
Generally
substances that cause depletion of ozone or make it thinner are called Ozone
Depletion Substances abbreviated as ODS. The loss of ozone molecules in the
upper atmosphere is termed as depletion of stratospheric ozone.
Oxides of Nitrogen:
Nitrogen
oxides introduced directly into the stratosphere by the supersonic jet aircraft
engines in the form of exhaust gases.
These
oxides are also released by combustion of fossil fuels and nitrogen
fertilizers. Inert nitrous oxide in the stratosphere is photo chemically
converted into more reactive nitric oxide. Oxides of nitrogen catalyse the
decomposition of ozone and are themselves regenerated. Ozone gets depleted as
shown below.
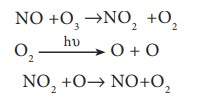
Thus
NO is regenerated in the chain reaction.
Chloro Fluoro Carbons (CFC) Freons
The
chloro fluoro derivatives of methane and ethane are referred by trade name
Freons. These Chloro Fluoro Carbon compounds are stable, non- toxic,
noncorrosive and non-inflammable, easily liquefiable and are used in
refrigerators, air- conditioners and in the production of plastic foams. CFC’s
are the exhaust of supersonic air craft’s and jumbo jets flying in the upper
atmosphere. They slowly pass from troposphere to stratosphere. They stay for
very longer period of 50 – 100 years. In the presence of uv radiation, CFC’s
break up into chlorine free radical
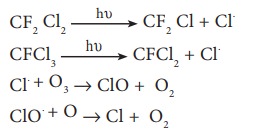
Chlorine
radical is regenerated in the course of reaction. Due to this continuous attack
of Cl˚ thinning of ozone layer takes place which leads to formation of ozone
hole.
It
is estimated that for every reactive chlorine atom generated in the
stratosphere 1,00,000 molecules of ozone are depleted.
Environmental Impact of Ozone Depletion
The
formation and destruction of ozone is a regular natural process, which never
disturbs the equilibrium level of ozone in the stratosphere. Any change in the
equilibrium level of the ozone in the atmosphere will adversely affect life in
the biosphere in the following ways.
![]()
![]() Depletion of ozone layer will allow more UV rays to reach
the earth surface and layer would cause skin cancer and also decrease the
immunity level in human beings.
Depletion of ozone layer will allow more UV rays to reach
the earth surface and layer would cause skin cancer and also decrease the
immunity level in human beings.
UV
radiation affects plant proteins which leads to harmful mutation of cells.
UV
radiation affects the growth of phytoplankton, as a result ocean food chain is
disturbed and even damages the fish productivity.
Related Topics