Chapter: Pharmaceutical Drug Analysis: Errors In Pharmaceutical Analysis and Statistical Validation
Significant Figures
SIGNIFICANT FIGURES
Generally, significant figures may be defined as—“All digits* that are certain plus one which
contains some uncertainty are said to be significant figures”.
Examples :
(a) Burette
Reading : Burettes are mostly
graduated with the smallest graduation as 0.1 ml ; hence, while taking the
burette reading the figures 6.3 ml can be read off with ample certainty.
However, the second place of the decimal is normally estimated by arbitrarily
sub-dividing the smallest division into 10 equal parts. Consequently, the final
burette reading of 6.32 ml essentially contains three significant figures, of
which two are certain, and one with some uncertainty.
(b) Measuring Weights : In the two measured
quantities : 4.7350 g and 4.0082 g the zero is a significant figure ; whereas,
in the quantity 0.0065 kg the zeros are not significant figures. Thus, in the
latter instance the zeros only serve to locate the decimal point and, therefore,
may be eliminated completely by proper choice of units, e.g., 6.5 g. Moreover, the first two numbers do have five
significant figures, whilst 0.0065 only has two significant figures.
1. Computation Rules
The following computation rules are advocated to make
sure that a calculated result, arrived at either by addition and subtraction or
multiplication and division essentially contains only the number of ‘digits’
duly justified by the experimental data.
(a) Addition and Subtraction
In addition and subtraction, retain only as many decimal
places as appear in the number that has the fewest decimals.
Example : Add algebraically the numbers
given : 16.48 + 9.375 – 3.5450 + 118.9.
Following three
steps are to be carried out sequentially :
(i) All numbers
are required to be rounded up preliminarily to two decimal places,
(ii) Add the rounded numbers, and
(iii) Final
result is then rounded to one decimal place.
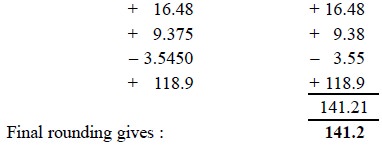
(b) Multiplication and Division
In multiplication or division, retain in each term one
more signifcant figure than is contained in the term with the largest
uncertainty. However, the percentage precision of product cannot be greater
than the percentage precision of the least precise term entering the
calculation. Hence, the multi-plication : 2.64 × 3.126 × 0.8524 × 32.9453 must
be accomplished using the values
2.64 × 3.126 × 0.852 × 32.95
which is equal to 231.6796. Thus, the result obtained may
be expressed to five significant figures as 231.68.
(c) Rounding Numbers
In rounding numbers, always drop the last digit in case
it is less than 5, e.g., 8.62 will
become 8.6. If the last digit is more than 5, always increase the preceeding
digit by one i.e., 9.38 will become
9.4. In case, the digit to be dropped is 5, always round up the preceding digit
to the nearest even number i.e., 8.75
will become 8.8 ; and 8.65 will become 8.6. Evidently, this method avoids a
tendency to round up numbers in one direction only.
In rounding off quantities to the nearest correct number
of significant figures, add one to the last figure retained provided the
following figure is either 5 or over. Hence, the average of 0.6526, 0.6521, and
0.6524 is 0.6525 (0.65237).
(d) Always
retain as many significant figures in a result as will yield only one uncertain
figure.
Examples : (i) A volume read off from a burette reading that lies between 15.6
ml and 15.8 ml must be recorded as 15.7 ml, but not as 15.70 ml, because the
latter would indicate that the reading lies between 15.69 and 15.71 ml.
(ii) A weight,
to the nearest 0.1 mg, is recorded as 2.4500 g ; and it must not be written as
either 2.450 g or 2.45 g, because in the latter instance an accuracy of a
centigram is emphasized whereas in the former a milligram.
Related Topics