Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Fishes as social animals: reproduction
Reproductive patterns among fishes
Reproductive patterns among fishes
Important components of a breeding system include frequency of mating, number of partners, and gender role of average individuals (Table 21.1). Fishes show greater diversity in these traits than do other vertebrates. Most fishes follow the mammalian and avian norm of remaining one gender throughout adult life, but many fish species change sex and some are parthenogenetic, producing young from unfertilized eggs. Some fishes retain a single mate, perhaps for life, others mate promiscuously, and a few are haremic. And in some fishes, a single breeding system may not characterize the entire species.

Lifetime reproductive opportunities
Most fishes are iteroparous, spawning more than once during their lives (e.g., sharks, lungfishes, sturgeons, gars, tarpons, minnows, trouts, codfishes, seabasses). However, some well-known species are semelparous, spawning one time and dying. Semelparity characterizes most salmon of the genus Oncorhynchus (e.g., Pink, Chum, Chinook, Coho, and Sockeye salmon). These fishes hatch in fresh water, migrate to the sea for a period of 1–4 years, and then return to their natal (birth) stream where they spawn and die. Although the life cycle of females appears to be relatively fixed across a species, intrapopulational
Table 21.1
A summary of components of breeding systems in fishes, with representative taxa. Accurate categorization is often hampered by the difficulty of following individual fish over extended periods in the wild. Although families are listed for some components, exceptions are common within a family. Modified from Wootton (1990), used with permission.
differences in male cycles are common (see below, Alternative mating systems and tactics).
Other semelparous fishes include lampreys, anguillid (freshwater) eels, and the osmeriform southern smelts (Retropinnidae) and galaxiids of Australia and New Zealand (McDowall 1987). American Shad (Alosa sapidissima) are semelparous in southern locations (30–33°N), largely iteroparous at northern latitudes (41–47°N), and variably iteroparous at intermediate latitudes (Leggett & Carscadden 1978). With the exception of such annual fishes as aplocheiloid rivulines, semelparous fishes are diadromous or include at least one major migratory phase in their life cycle. Anguillid eels show sex-based differences in tactics within an overall semelparous strategy. Males often mature rapidly (c. 3–6 years) and at a uniformly small size (30– 45 cm) regardless of locale, whereas females are consistently longer (35–100 cm) and may mature quickly (4–13 years) at low latitudes or slowly (6–43 years) at high latitudes. Slow maturing females grow larger and produce more eggs than smaller, faster maturing females. In American eels, males have a relatively restricted geographic distribution, occurring primarily in estuaries of the southeastern United States, whereas females are found throughout the North American range of the species and in all habitats. As far as is known, all members of an anguillid species migrate to the same oceanic region to spawn and die (Sargasso Sea in the western Atlantic for American and European eels, the Philippine Sea for Japanese eels) (Helfman et al. 1987; Jessop 1987; Representative life histories of migratory fishes).
Mating systems
Mating systems are defined by the number of mating partners an individual has during a breeding season (Table 21.1). The three most common categories are promiscuous, polygamous, and monogamous. Promiscuous breeders are those in which little or no obvious mate choice occurs, where both males and females spawn with multiple partners, either at one time or over a short period. Such spawning has been documented for the Baltic Herring (Clupeidae), Guppies (Poeciliidae), Nassau Groupers (Serranidae),humbug damselfish colonies (Pomacentridae), cichlids, and the Creole Wrasse (Labridae) (Thresher 1984; Barlow 1991; Turner 1993).
Polygamy, where only one sex has multiple partners, takes multiple forms. Polyandry, where one female mates with several males (and presumably not vice versa), is relatively uncommon, so far documented only in an anemonefish (Pomacentridae) (Moyer & Sawyers 1973). Polyandry might also be descriptive of female ceratioid anglerfishes which have more than one male attached (see The deep sea). Polygyny is the most common form, involving males as the polygamous sex. Territorial males that care for eggs and young are frequently visited by several females, as in sculpins, sunfishes, darters, damselfishes, and cichlids. Polygyny can also develop into harem formation, where a male has exclusive breeding rights to a number of females that he may guard. Harems have been observed in numerous cichlids and in several coral reef families (e.g., tilefishes, anthiine serranids, damselfishes, wrasses, parrotfishes, surgeonfishes, triggerfishes).
Many polygynous animals form leks, which are traditional areas where several males congregate for the sole purpose of displaying to females (Emlen & Oring 1977). Females are often attracted to a male in response to his central position within the lekking ground, or to the vigor of his display and bright plumage. Lekking is common in birds and mammals, in which only the female provides parental care. Some African cichlids come closest to forming true leks. Large numbers (c. 50,000) of maleCyrtocara eucinostomus congregate along a shallow 4 km long shelf in Lake Malawi and build sand nests and display to passing females each morning. Females spawn and then mouthbrood eggs elsewhere. The male aggregations break up each afternoon, when fish feed (McKaye 1983, 1991). Some fishes form “leklike” aggregations of males (e.g., Arctic Char, Atlantic Cod, damselfishes, wrasses, parrotfishes, surgeonfishes), but the display ground is also an appropriate place for launching or caring for eggs, which stretches the definition of lekking (Loiselle & Barlow 1978; Moyer & Yogo 1982; Figenschou et al. 2004; Windle & Rose 2007). In a unique variation on leklike behavior, female triggerfish (Odonus niger, Balistidae) form a communal display ground for 1 day before spawning, after which they all mate with a single, nearby male (Fricke 1980).
In monogamous systems, fish live in pairs that stay together and mate, or mate with the same individual repeatedly and exclusively, regardless of pairing at nonmating times. Strongly pairing species include North American freshwater catfishes, many butterfl yfishes and angelfishes, most substrate guarding and some mouthbrooding cichlids, and anemonefishes; in the butterfl yfishes, pairs may remain together for several years and probably mate for life (Reese 1975). Monogamous coral reef fishes commonly spawn with the same partner on a daily basis over an extended period without ensuing care of young, whereas freshwater species such as cichlids spawn over a limited time and then both parents typically care for the young. Monogamy has evolved independently in many groups, often in conjunction with territoriality and paternal care (Whiteman & Côté 2004). Monogamy is also known in freshwater bonytongues, bagrid and airsac catfishes, and snakeheads, and among at least 18 marine families, including pipefishes and seahorses, hermaphroditic hamlets, jawfishes, cardinalfishes, tilefishes, hawkfishes, damselfishes, wrasses, blennies, gobies, wormfishes, surgeonfishes, triggerfishes, filefishes, and pufferfishes (Barlow 1984, 1986; Thresher 1984; Turner 1993, Whiteman & Côté 2004).
Gender roles in fishes
Although the vast majority of fishes are gonochoristic, with sex determined at an early age and remaining fixed as male or female, a significant number of fishes can function as males or females simultaneously or sequentially. The environmental correlates and evolutionary causes of sex change in fishes have been the subject of considerable study and speculation.
Sex reversal has evolved, apparently independently, in perhaps 34 families belonging to 10 orders, including moray eels (Anguilliformes), loaches (Cypriniformes), lightfishes (Stomiiformes), killifishes (Atheriniformes), swamp eels (Synbranchiformes), flatheads (Scorpaeniformes), boxfishes (Tetraodontiformes), and at least 24 perciform families (including snooks, seabasses, tilefishes, emperors, rovers, porgies, threadfins, angelfishes, bandfishes, damselfishes, wrasses, parrotfishes, and gobies) (Devlin & Nagahama 2002; DeMartini & Sikkel 2006). Sex changers can be either: (i) simultaneous hermaphrodites, capable of releasing viable eggs or sperm during the same spawning; or (ii) sequential hermaphrodites, functioning as males during one life phase, and as females during another. Among sequential hermaphrodites,protandrous fishes develop first as males and then later change to females, whereas protogynous fishes mature first as females and then later become males. Variations on these patterns exist, such as protogynous populations with some males that develop directly from juveniles, or simultaneous hermaphrodites that lose the ability to function as one sex (Smith 1975; Warner 1978; Sadovy & Shapiro 1987; Lutnesky 1994).
Protogyny is by far the most common form of hermaphroditism, exhibited in at least 17 tropical marine families, which is about one-fi fth of reef families (DeMartini & Sikkel 2006). In a classic study, Robertson (1972) found that the Indo-Pacific Cleaner Wrasse, Labroides dimidiatus, formed harems of one large male and up to 10 females. Breeding access to the male was determined by a behavioral dominance hierarchy or peck order, the largest female dominating the next smallest and so on. If the top (alpha) female was removed, the next largest female assumed her role and everyone else moved up a step. If the male was removed, the alpha female began courting females within an hour and developed functional testes within 2 weeks (see also Kuwamura 1984).
Protogyny in wrasses can take other forms. In the Caribbean Bluehead Wrasse, Thalassoma bifasciatum, fish usually begin life as predominantly yellow females or similarly colored males (“initial phase” coloration). Any of the initial phase fish can change into larger, “terminal phase” males, which also develop a blue head, a black-and-white midbody saddle, and a green posterior region. Large males set up territories over coral heads that females prefer as spawning sites. Some females are intercepted by and spawn with groups of up to 15 smaller males, but the largest, pairspawning males have the highest spawning success. A territory- holding male may receive 40–100 spawnings per day, whereas a nearby group-spawning male may receive only one to two matings, and his sperm will often be diluted by the gamete output of other males in the group (Warner et al. 1975; Warner 1991). Other well-studied protogynous species include the anthiine serranid, Anthias squamipinnis, a pair-spawning species that forms large aggregations in which females may outnumber males by 36 : 1. The precision of social control of sex change in this species is remarkable: if nine males are removed from a large group, nine females change sex to replace them. Sex change to male in Anthias also occurs if the female : male ratio exceeds a threshold value (Shapiro 1979, 1987). The commonness of protogyny probably reflects the fact that most teleosts, including gonochoristic species, differentiate first as nonfunctional females.
Protandry has been reported in moray eels, loaches, lightfishes, platycephalids, snooks, porgies, threadfins, damselfishes, and crediid sandburrowers. The popular clown or anemonefishes (Amphiprion spp., Pomacentridae) live in groups of two large and several small individuals in an anemone. Only the two largest fish in an anemone are sexually mature, the largest individual being female and the next largest being male. Although smaller fish may be as old as the spawning individuals, the behavioral dominance of the mature pair keeps these smaller males from maturing and growing, and a dominance hierarchy exists among the smaller males. In essence, “low ranking males are psychophysiologically castrated” (Fricke & Fricke 1977, p. 830). If the female dies, the male changes sex to female and the next largest fish in the group takes over his former role and grows rapidly (Allen 1975; Moyer & Nakazano 1978). This inconvenient truth was judiciously sidestepped in the otherwise biologically accurate movie, Finding Nemo. In fact, Nemo’s dad, Marlin, should have become Nemo’s mother.
Simultaneous hermaphroditism (=cosexuality, synchronous hermaphroditism) is least common, known from only four shallow water families (muraenids, rivulids, serranids, gobies) and most of the 16 families in the deepsea order Aulopiformes (lizardfishes, Synodontidae, are the bestknown exception) (Smith 1975; Warner 1978; St. Mary 2000; Devlin & Nagahama 2002). Three species of New World cyprinodontiform rivulids are capable of selffertilization (Kryptolebias spp. of South America and the mangrove rivuline, Kryptolebias marmoratus, of North and Central America). Self-fertilization in Kryptolebias is internal, producing clonal populations of homozygous, genetically identical hermaphroditic fish. Functional males can be produced depending on temperature and day length (Harrington 1971, 1975; Taylor 1992). Cyprinodontiform fishes are often colonists of small streams on islands and other seasonally adverse habitats. Selffertilization may be one means of assuring mates in low density populations that frequently become isolated, a scenario that could also be applied to the deepsea aulopiforms.
The other species of simultaneous hermaphrodites occur among the small hamlets (Hypoplectrus, Serranus). Each individual is physiologically capable of producing sperm and eggs at the same time, but behaviorally these fishes function as only one sex at a time during a spawning bout. In Caribbean hamlets (Hypoplectrus), spawning bouts can last for several hours, during which time members of a pair alternate sex roles, one fish first behaving as the “female” and releasing eggs and then behaving as the “male” and releasing sperm (Pressley 1981; Fischer & Petersen 1987). The eastern Pacific Serranus fasciatus is a haremic, sexchanging, simultaneous hermaphrodite: one male guards and spawns with several hermaphrodites that act as females. If the male is removed, the largest hermaphrodite changes into a male (Fischer & Petersen 1987). Serranines have separate external openings for the release of eggs and sperm (in addition to an anus), which may prevent internal or accidental self-fertilization. Self-fertilization may occur in some serranines, but only in aquaria (Thresher 1984).
One additional group of fishes departs from normal gonochoristic gender roles. Livebearers in Mexico and Texas include parthenogenetic “species” that are all-female but require the sperm from males of other species to activate cell division in their eggs. Parthenogenesis in livebearers takes two forms: gynogenesis and hybridogenesis (Fig. 21.1). Gynogenetic females are usually triploid and produce eggs that are also 3N. These eggs are activated by sperm from other species, but no sperm material is incorporated; hence daughters are genetically identical to their mothers. Hybridogenetic females, in contrast, are diploid and produce haploid eggs that, during the reduction division of meiosis, keep the maternal genes and discard the paternal genes. Upon mating, these eggs unite with sperm from males of another species, forming a new, diploid hybrid daughter (no sons are produced). When the daughter mates, she again produces eggs that are haploid and “female”. Hence the maternal lineage is conserved and the male’s genetic contribution is lost after one generation. These parthenogenetic “species” are thought to have arisen originally as hybrids betweenPoeciliopsis monacha females and males of four congeners, P. lucida, P. occidentalis, P. latidens, and P. viriosa. The males of the four species are the usual sperm donors during mating. An additional species, the Amazon Molly, Poecilia formosa, is diploid and gynogenetic. Sperm from two other species (P. mexicana and P. latipinna) activate the eggs, but contribute no genetic material (Schultz 1971, 1977; Vrijenhoek 1984). Natural gynogenesis has also been reported for the cyprinid Cyprinus auratus gibelio (Price 1984).
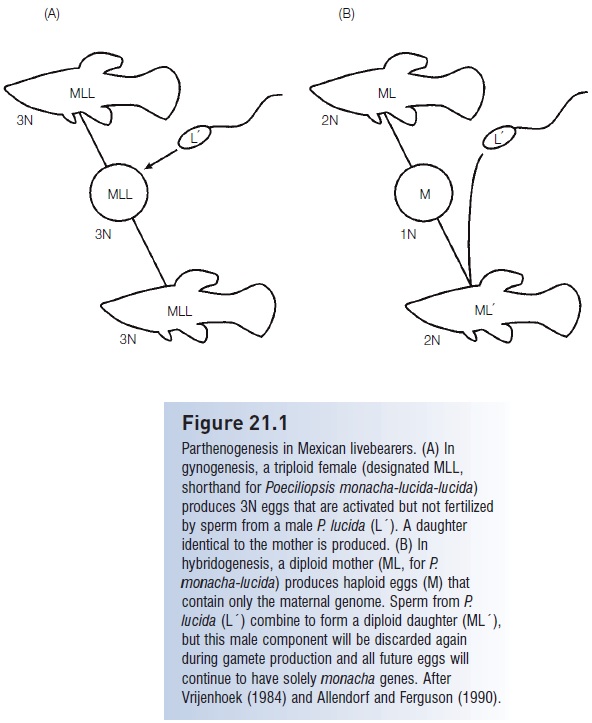
Figure 21.1
Parthenogenesis in Mexican livebearers. (A) In gynogenesis, a triploid female (designated MLL, shorthand for Poeciliopsis monacha-lucida-lucida) produces 3N eggs that are activated but not fertilized by sperm from a male P. lucida (L´). A daughter identical to the mother is produced. (B) In hybridogenesis, a diploid mother (ML, for P. monacha-lucida) produces haploid eggs (M) that contain only the maternal genome. Sperm from P. lucida (L´) combine to form a diploid daughter (ML´), but this male component will be discarded again during gamete production and all future eggs will continue to have solely monacha genes. After Vrijenhoek (1984) and Allendorf and Ferguson (1990).
An immediate question that arises is how natural selection maintains males that waste gametes so wantonly. Apparently, dominance hierarchies among “donor male” populations of live-bearers exclude many males from mating with conspecific females. These are often the males that participate in the parasitized, heterospecific spawnings. Satellite or peripheral males that have very low reproductive output are characteristic of many vertebrate species (these are often the sneakers and streakers discussed below), providing an abundance of otherwise unused sperm (Moore 1984). Additionally, laboratory tests of mate preferences in sexual females show that sexual females are more attracted to males that the females observed courting gynogenetic females. Apparently a male can increase his chances of mating with a sexual female if he spends time courting asexual females because sexual females copy the choices made by female gynogens. It is not known whether sexual females prefer males that mate with sexual females over those that mate with gynogenetic females (Schlupp et al. 1994).
Certain generalities arise from surveys of sex change in shallow water fishes, as do exceptions. Sex change is largely a tropical and subtropical, marine phenomenon (Policansky 1982c; Warner 1982). Cool temperate marine and freshwater sex changers are known (e.g., loaches, bristlemouths, swamp eels, wrasses, gobies) but are relatively uncommon compared with tropical marine hermaphrodites. Patterns often follow familial lines, all members of a family being either protandrous or protogynous (although there are both protogynous and protandrous species among moray eels, seabasses, porgies, damselfishes, and gobies, and some serranids are clearly simultaneously hermaphroditic).
However, population differences are becoming increasingly well known in sex-changing fishes. The Cleaner Wrasse, Labroides dimidiatus, is haremic under some conditions but forms pairs under others. Bluehead Wrasse, Thalassoma bifasciatum, are dominated by territorial-spawning males on small reefs with small populations, but by groupspawning males on large reefs with dense populations. Resource limitation, either food availability or reef size, and population size are frequent determinants of variation in mating systems. Clearly, sex change and mating systems respond to environmental variability (see Thresher 1984; Shapiro 1991; Warner 1991; Devlin & Nagahama 2002; Oldfield 2005).
Related Topics