Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Fishes as social animals: reproduction
The gender of care-givers - Parental care of Fishes
The gender of care-givers
The most common care-giver in fishes is the male. Males alone or in combination with females (=biparental care) account for approximately 80% of 77 families in which the sex of the care-giver is known; males alone care for young in 36–39 families (Blumer 1979, 1982; Mank et al. 2005). The predominance of male parental care in fishes contrasts markedly with its occurrence in other vertebrates, where care by females (amphibians, mammals) or both parents (birds) is more common (post-hatching care is uncommon in reptiles). Male guarding may be explainable as an evolutionary result of external fertilization and a male’s way of assuring he alone fertilizes a batch of eggs (=paternity assurance) (Ah-King et al. 2005). To accomplish this, a male should: (i) provide a suitable locale where females will lay eggs to be fertilized; and (ii) guard the eggs so that no other male can fertilize them.
Paternity assurance was the likely driving force behind the evolution of brood pouches in pipefishes and seahorses.
The female deposits eggs in the male’s abdominal pouch, where only his sperm are likely to reach the eggs. In most species, extending care beyond the fertilization stage greatly increases the probability of successful hatching and dispersal, thereby increasing the likelihood that offspring will live to reproduce. Egg and larval predators are common in all environments, as are fungal infections. A guarding male can chase off fishes and invertebrates that might eat the eggs, and can remove diseased or dead eggs, thus slowing the spread of fungi and other infectious pathogens.
Males may care for young longer because females are more likely to spawn with males that already have eggs or young in the nest (e.g., Fathead Minnow, Three-spined Stickleback, Painted Greenling, River Bullhead, Tessellated Darter, Browncheek Blenny). An unexpected outcome of a female preference for males with eggs is nest and egg usurpation (also known as allopaternal oralloparental care). Male sticklebacks will raid other males’ nests, steal eggs, and deposit these eggs in their own nest. Male Fathead Minnows evict males from existing nests and then guard the acquired eggs. In brood piracy, a large male may usurp the nest of another male, spawn, and then abandon the nest to be guarded by the original territory holder (Van den Berghe 1988; Magnhagen 1992).
A related phenomenon is interspecific brood parasitism or egg dumping, where one species spawns in a nest constructed by and guarded by another species. Several species, including gars and minnows, spawn in nests guarded by male sunfishes, and Golden Shiner are known to spawn in nests of Bowfin and Largemouth Bass. Small minnows also spawn over the mound nests built by larger minnows, such as Bluehead Chub (see above); the eggs are guarded by the large male chub. The chub may benefit by a dilution effect whereby predators are likely to eat the more numerous minnow eggs, whereas the minnow eggs receive the protection of a nest guarded by a large male. A dilution effect probably explains why bagrid catfish tolerate and guard cichlid young in their nests. Mistaken identity cannot be invoked, since the guarding catfish parents selectively chase cichlid young to the periphery of the nest, exposing the cichlids to higher predation rates and decreasing mortality in the catfish young. The young cichlids benefit from the protection of two large catfish plus the mother cichlid that remains nearby (McKaye 1981b; Unger & Sargent 1988; McKaye et al. 1992).
Other examples of brood parasitism include the mochokid Cuckoo Catfish, Synodontis multipunctatus, of Lake Tanganyika, which parasitizes broods of several mouth-brooding cichlids by laying its eggs on the substrate as the female cichlid is picking up her own fertilized eggs. The young catfishes eventually eat the cichlid larvae (Sato 1986; Barlow 2000) (Fig. 21.12). Clariid catfishes in Lake Tanganyika are known to dump eggs in nests of auchenoglanidid catfishes (Ochi et al. 2001), and mixed species brooding has been observed in Lake Baikal sculpins (Munehara et al. 2002).
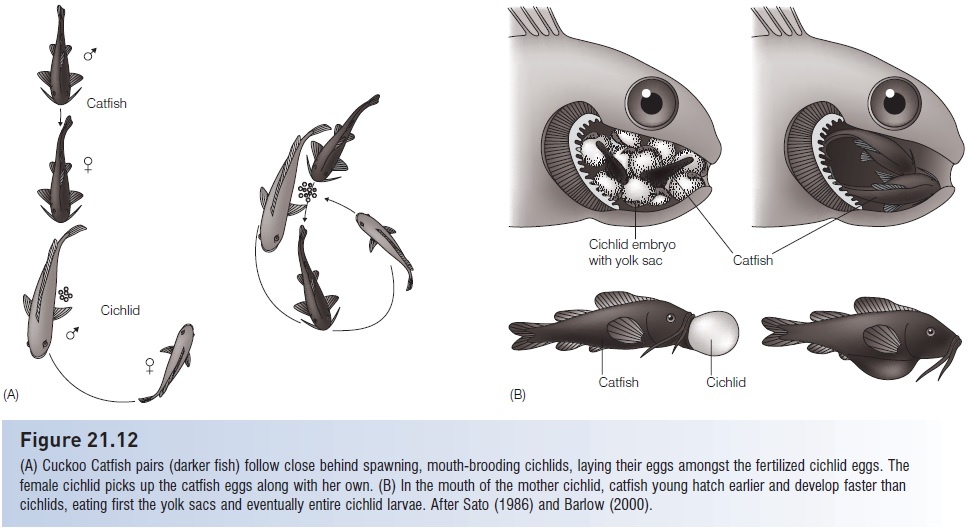
Figure 21.12
(A) Cuckoo Catfish pairs (darker fish) follow close behind spawning, mouth-brooding cichlids, laying their eggs amongst the fertilized cichlid eggs. The female cichlid picks up the catfish eggs along with her own. (B) In the mouth of the mother cichlid, catfish young hatch earlier and develop faster than cichlids, eating first the yolk sacs and eventually entire cichlid larvae. After Sato (1986) and Barlow (2000).
Brood parasitism carries obvious potential costs, as evidenced by the Cuckoo Catfish example, so it is not surprising that some species have tactics that apparently counteract such parasites.
A Japanese fish, the taxonomically uncertain Aucha Perch, Siniperca(or Coreoperca) kawamebari (Percichthyidae?), is parasitized by a native minnow with a shorter spawning season; egg dumping leads to higher predation rates on perch eggs. Female Aucha Perch, which normally prefer to spawn in nests with more eggs, avoid perch nests with high numbers of eggs during the minnow’s spawning season (Baba & Karino 1998).
If male care evolved to insure that no other males fertilized the eggs, then males would not be expected to provide care in 21 teleostean families with internal fertilization (e.g., Mank et al. 2005). This is almost universally true and even applies to species that are exceptional relative to the familial norm. For example, most sculpins have external fertilization and male parental care. InClinocottus analis and Oligocottus spp., fertilization is internal and male parental care is absent (Perrone & Zaret 1979). A card inalfish, Apogon
Spawning occurs repeatedly over an extended 5-day period, the male chases off other males, and he also picks up eggs in his mouth immediately after they are laid, all actions that would minimize the opportunity for other males to fertilize the eggs (Blumer 1979).
A final category of care deserving attention is the phenomenon of cooperative breeding or helpers at the nest. Nonparental care-givers, usually young from a previous breeding episode, remain with the parents and feed and defend new young or defend and maintain the territory. Such helpers occur in over 150 bird and 25 mammal species and in at least 19 species of Lake Tanganyika cichlids (Taborsky 1984; Heg & Bachar 2006). Two of the beststudied Tanganyika species are Neolamprologus pulcher andLamprologus brichardi (e.g., Stiver et al. 2006). In L. brichardi, helpers remain for about a year through two to three subsequent breeding cycles. They clean and fan eggs, larvae, and fry, remove sand and snails from the breeding hole, and defend the parental territory. Helpers suffer slower growth rates than nonhelping individuals, but receive protection from predators due to territorial shelters and the protective activities of larger family members. Females with helpers produce more fry.
Helping generally imposes a cost because helpers do not reproduce directly while remaining with their parents. However, helpers may promote their fitness (contribution of genes to future generations) more by raising siblings to whom they are closely related than by attempting to breed on their own. Helping is thus an example where kin selection explains an apparently altruistic activity. It is somewhat remarkable that cooperative breeding in fishes has as yet only been observed in related cichlids in Lake Tanganyika (helpers among anemonefishes are suspected but to date been found lacking, e.g., Buston 2004). Cooperative breeding among cichlids is additional evidence of the tremendous ecological and evolutionary plasticity of that family.
Related Topics