Chapter: Biology of Disease: The Nature and Investigation of Diseases
Quality of Test Results and Clinical Auditing
QUALITY OF TEST RESULTS AND
CLINICAL AUDITING
The results of tests performed in pathology
laboratories assist with diagnosis of disease or the monitoring of treatment.
Thus they can greatly influence the management of patients, and it is essential
to assure the quality of laboratory results. Erroneous results have the
potential to cause considerable harm (both physical and psychological) to
patients and must be avoided. All laboratories have practices and procedures to
ensure erroneous results are minimized and that good quality results are
provided. Errors can arise at the three different stages of analysis, that is,
preanalytical, analytical and postanalytical. Preanalytical errors occur before the sample has been analyzed.
Analytical errors arise during the laboratory testing procedure. Postanalytical
errors arise after the specimen has been analyzed.
In general, preanalytical mistakes result from
inappropriate methods of collection or incorrect labeling, handling, transport
or storage of the specimen. Experimental mistakes that can give rise to
analytical errors are detected by introducing systems for each clinical test to
warn when errors occur. This is normally achieved by analyzing a control sample within each batch of
tests. A control sample is one that is identical in composition to the test
samples except that it contains a known concentration of the test analyte. All
samples, including the control sample, must be treated identically. For
example, if the concentration of glucose in serum is being determined, then the
control should be serum, not water, containing a known concentration of
glucose.
The control sample is usually an aliquot from a
larger sample for which the mean and standard deviation have already been
determined. The results for control samples are usually recorded graphically so
that changes in the quality of the method are detected as soon as they arise. A
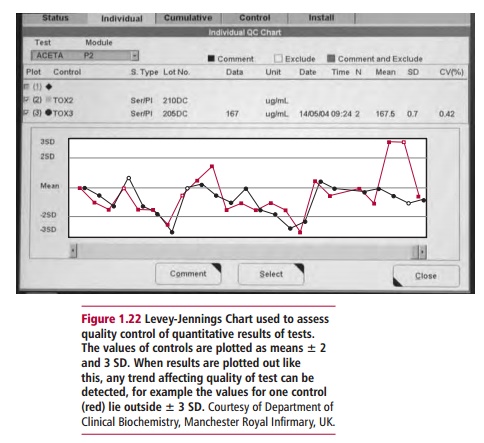
common chart used for quality control purposes is the Levey-Jennings chart (Figure 1.22) in which the control limits
are set at the mean ± 2 SD and ± 3 SD. If the control values fall outside the ±
2 SD limit, that is, there is drift away from the accepted limits, there is
only a 5% probability that the result lies in a normal distribution around the
mean and is still valid. Any results that lie outside the 3 SD warning limit
suggests that a problem is occurring with the method. Problems could include
unstable reagents in the analyzer, problems with temperature control or
contamination, all of which require investigation. Occasionally there are gross
(and usually very obvious) inaccuracies in the value of a test result such that
it bears no resemblance to values seen in health or disease. These are referred
to as ‘blunders’. Blunders usually arise because of transcriptional errors in
reporting the result. To reduce the number of blunders, results should be
checked thoroughly by senior staff before being sent to clinicians.
Most laboratories have their own quality control
samples and these are used for internal quality assurance purposes. Many
countries now participate in external quality assurance, whereby quality
control samples are sent to participating laboratories from a central source to
assess the analytical performance of their methods for particular analytes.
Furthermore, to ensure quality of service provision, many laboratories follow a
set of procedures required for accreditation by external agencies. These
procedures ensure good laboratory practice (GLP) and cover all aspects of the
laboratory that are involved in the production of test results. These
procedures ensure that all laboratory staff are adequately trained and have
clearly defined responsibilities. The equipment used should be of adequate
standard with a logbook showing a full record of maintenance and faults. All
methods used in the laboratory are standardized, fully documented and
appropriate for the analysis. Full details of each method are provided as a
standard operating procedure (SOP) that includes details of specimen handling,
the analytical method, equipment used and quality control procedures.
To improve the quality of the services they provide,
many laboratories participate in some form of audit. Clinical audit (Figure 1.23)
is a process whereby practices and procedures involved in patient care are
monitored and, if necessary, revised to provide a more efficient and
cost-effective service that should ultimately benefit the patient. Audit is
part of the process of ensuring
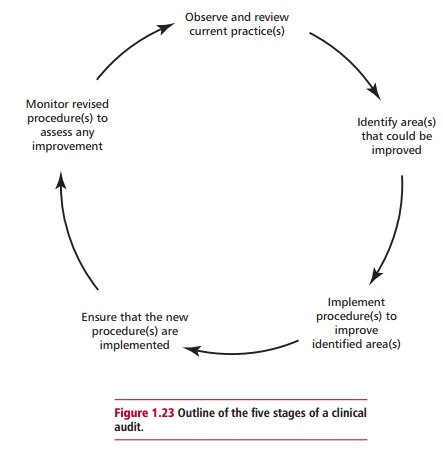
quality and is usually divided into five stages. The
first is the observation and review of current practice and procedures in the
laboratory. The second stage involves the identification of areas of concern
that could be improved and questions are asked as to whether the current
service can be provided more economically. Third, a series of changes are
devised to rectify and improve the identified area(s) of concern. Fourth, these
changes are implemented and steps taken to ensure compliance and, finally, at
the fifth stage, the changes are monitored and compared with previous
procedures in order to assess whether there is indeed any improvement in the
service provided or whether the revised procedures are actually more cost
effective.
A clinical audit is usually followed by a re-audit
after an appropriate period of time. The audit may include several processes
such as the initial stages of test requesting, specimen collection and
transport. The audit may wish to investigate whether appropriate advice is
available to clinicians requesting tests, whether the test request forms are
easy to use or whether appropriate containers are provided for specimen
collection. Other types of audit processes may relate to the analytical service
provided by the laboratory, such as whether the repertoire of tests offered is
appropriate to the needs of the clinical service. A clinical audit may wish to
investigate whether provision of the laboratory service out of hours is efficient
and cost-effective and whether test results are being returned to the
clinicians at the right place and within an appropriate time.
Related Topics