Chapter: Biochemistry: The Metabolism of Nitrogen
Purine Biosynthesis
Purine Biosynthesis
We have already discussed the formation of ribose-5-phosphate as
part of the pentose phosphate pathway. The biosynthetic pathway for both purine
and pyrimidine nucleotides uses preformed ribose-5-phosphate. Purines and
pyrimidines are synthesized in different ways, and we shall consider them separately.
Anabolism of Inosine Monophosphate
In the
synthesis of purine nucleotides, the growing ring system is bonded to the
ribose phosphate while the purine skeleton is being assembled—first the
five-membered ring and then the six-membered ring—eventually producing
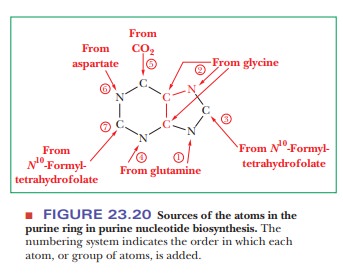
inosine-5'-monophosphate. All four nitrogen atoms of the purine ring are
derived from amino acids: two from glutamine, one from aspartate, and one from
glycine. Two of the five carbon atoms (adjacent to the glycine nitrogen) also come
from glycine, two more come from tetrahydrofolate derivatives, and the fifth
comes from CO2 (Figure 23.20). The series of reactions producing
inosine monophosphate (IMP) is long and complex.
How is inosine monophosphate convertedto AMP and GMP?
IMP is
the precursor of both AMP and GMP. The conversion of IMP to AMP takes place in
two stages (Figure 23.21). The first step is the reaction of aspartate with IMP
to form adenylosuccinate. This reaction is catalyzed by adenylosuccinate
synthetase and requires GTP, not ATP, as an energy source (using ATP would be
counterproductive). The cleavage of fumarate from adenylosuccinate to produce
AMP is catalyzed by adenylosuccinase. This enzyme also functions in the
synthesis of the six-membered ring of IMP.
The conversion of IMP to GMP also takes place in two stages (Figure 23.21). The first of the two steps is an oxidation in which the C—H group at the C-2 position is converted to a keto group. The oxidizing agent in the reaction is NAD+, and the enzyme involved is IMP dehydrogenase. The nucleotide formed by the oxidation reaction is xanthosine-5'-phosphate (XMP).
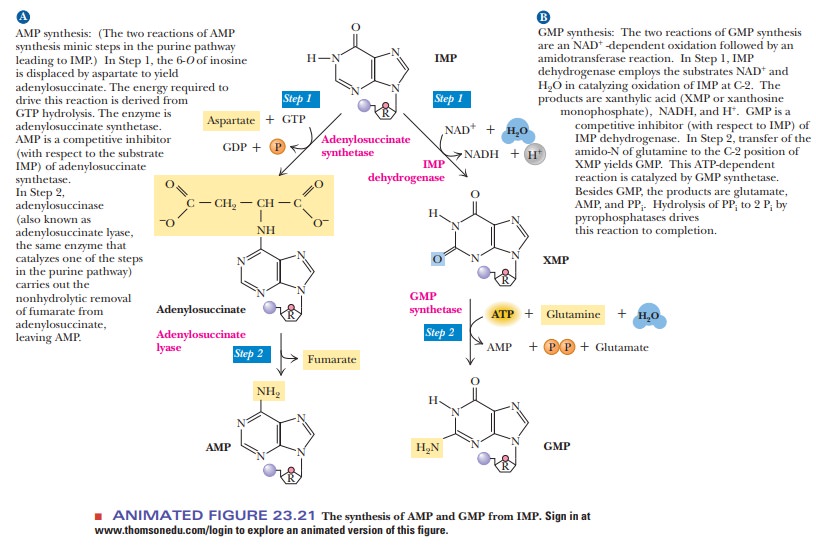
An amino group from the
side chain of glutamine replaces the C-2 keto group of XMP to pro-duce GMP.
This reaction is catalyzed by GMP synthetase; ATP is hydrolyzed to AMP and PPi
in the process. Note that there is some control over the relative levels of
purine nucleotides; GTP is needed for the synthesis of adenine nucleo-tides,
whereas ATP is required for the synthesis of guanine nucleotides. Each of the
purine nucleotides must occur at a reasonably high level for the other to be
synthesized.
Subsequent
phosphorylation reactions produce purine nucleoside diphos-phates (ADP and GDP)
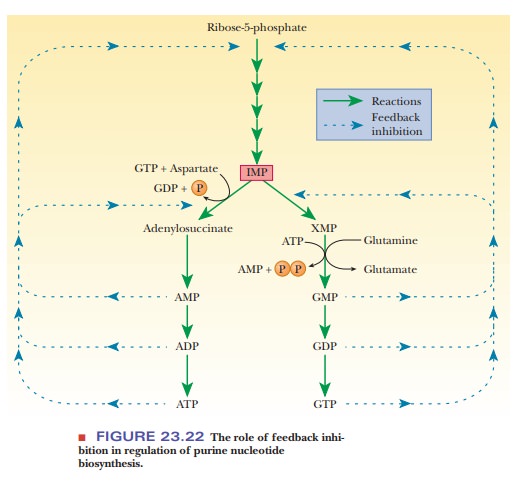
and triphosphates (ATP and GTP). The purine nucleoside monophosphates,
diphosphates, and triphosphates are all feedback inhibitors of the first stages
of their own biosynthesis. Also, AMP, ADP, and ATP inhibit the conversion of
IMP to adenine nucleotides, and GMP, GDP, and GTP inhibit the conversion of IMP
to xanthylate and to guanine nucleotides (Figure 23.22).
What are the energy requirements for production of AMP and GMP?
The production of IMP starting with ribose-5-phosphate requires the equivalent of 7 ATP. The conversion of IMP to AMP requires hydrolysis of an additional high-energy bond—in this case, that of GTP. In the formation of AMP from ribose-5-phosphate, the equivalent of 8 ATP is needed. The conversion of IMP to GMP requires two high-energy bonds, given that a reaction occurs in which ATP is hydrolyzed to AMP and PPi. For the production of GMP from ribose-5-phosphate, the equivalent of 9 ATP is necessary. The anaerobic oxidation of glucose produces only 2 ATP for each molecule of glucose. Anaerobic organisms require four molecules of glucose (which produce 8 ATP) for each AMP they form, or five molecules of glucose (which produce 10 ATP) for each GMP.
The process is more efficient for aerobic organisms. Since 30 or 32 ATP result from each molecule of glucose, depending
on the type of tissue, aerobic organisms can optimally produce four AMP
(requiring 32 ATP) or three GMP (requiring 36 ATP) for each molecule of glucose
oxidized. A mechanism for reuse of purines, rather than complete turnover and
new synthesis, saves energy for organisms.
Summary
The growing ring system of purines is attached to ribose phosphate
during the synthesis process.
The biosynthesis of nucleotides requires considerable expenditures
of energy by organisms in long and complex pathways. Feedback inhibition at all
stages plays a key role in regulating the pathway.
Related Topics