Chapter: Biochemistry: The Metabolism of Nitrogen
Amino Acid Biosynthesis
Amino Acid Biosynthesis
Ammonia is toxic in high concentrations, and so it must be
incorporated into biologically useful compounds when it is formed by the
reactions of nitrogen fixation discussed earlier. The amino acids glutamate and
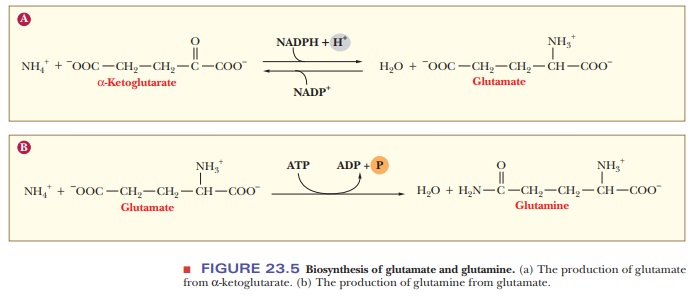
glutamine are of central importance in the process. Glutamate arises from α-ketoglutarate, andglutamineis formed from glutamate (Figure 23.5).The production of
glutamate is a reductive amination, and the production of glutamine is
amidation. In other reactions of amino acid anabolism the α-amino group of glutamate and
the side-chain amino group of glutamine areshifted to other compounds in transamination reactions.
What are some common features in amino acid biosynthesis?
The biosynthesis of amino acids involves a common set of reactions.
In addition to transamination reactions, transfer of one-carbon units, such as
formyl or methyl groups, occurs frequently. We are not going to discuss all the
details of the reactions that give rise to amino acids. We can, however,
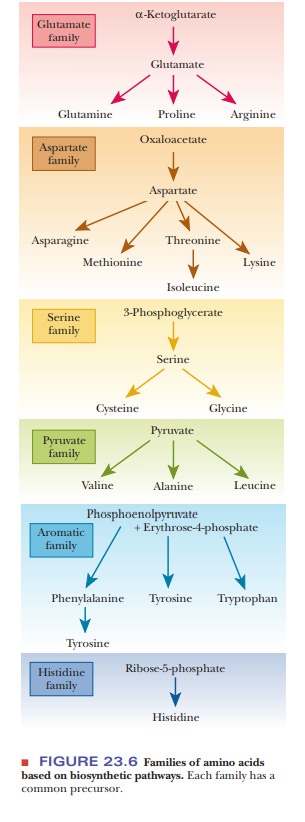
organize this material by grouping amino acids into families based on common
precursors (Figure 23.6). The reactions of some of the individual families of
amino acids provide good examples of reactions that are of general importance,
such as transamination and one-carbon transfer.
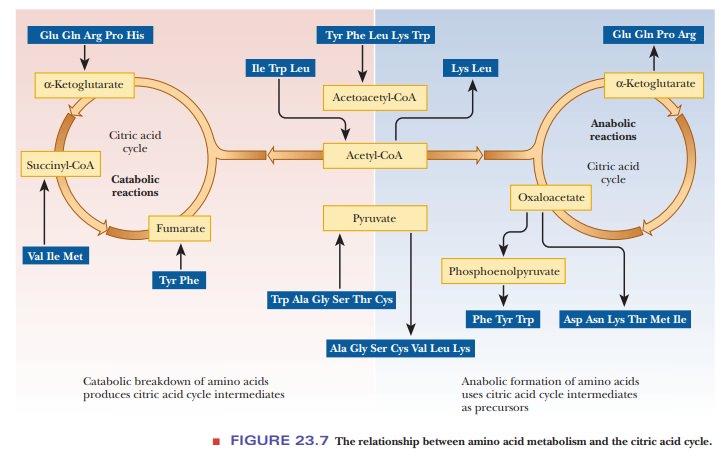
We can also make some generalizations about amino acid metabolism
in terms of the relationship of the carbon skeleton to the citric acid cycle
and the related reactions of pyruvate and acetyl-CoA (Figure 23.7). The citric
acid cycle is amphibolic; it has a part in both catabolism and anabolism. The
anabolic aspect of the citric acid cycle is of interest in amino acid
biosynthesis. The cata-bolic aspect is apparent in the breakdown of amino
acids, leading to their even-tual excretion, which takes place in reactions
related to the citric acid cycle.
What makes transamination reactions important in amino acid biosynthesis?
Glutamate is formed from NH4+ and α-ketoglutarate in a reductive amination that requires NADPH. This reaction is reversible and is catalyzed by glutamatedehydrogenase (GDH).
Glutamate
is a major donor of amino groups in reactions, and α-ketoglutarate is a major acceptor of amino groups (see Figure
23.5a). Note the requirement for reducing power.
NH4+
+ α-ketoglutarate + NADPH + H+ - > Glutamate + NADP+
+ H2O
The
conversion of glutamate to glutamine is catalyzed by glutamine synthe-tase (GS) in a reaction that requires ATP (see
Figure 23.5b).
NH4+
+ Glutamate + ATP - > Glutamine + ADP + Pi + H2O
These reactions fix inorganic nitrogen (NH3), forming organic (carbon-containing) nitrogen compounds, such as amino acids, but they frequently do not operate in this sequential fashion.
In fact, the combination of GDH and GS
is responsible for most of the assimilation of ammonia into organic compounds,
especially in organisms that are rich in nitrogen sources. However, the KM of GS is considerably
lower than that of GDH. When nitrogen is limiting, as is frequently the case in
plants, the conversion of glutamate to glutamine is the preferred mode of
nitrogen assimilation. This means that the supply of glutamate becomes depleted
unless there is some way to replenish it. The reductive amination of α-ketoglutarate with the amide nitrogen of glutamine as the nitrogen
source is the way this is done.
Reductant + α-Ketoglutarate + Glutamine - > 2 Glutamate + Oxidized reductant
The
reductant can be NADH, NADPH (in yeast and bacteria), or reduced ferredoxin (in
plants). The enzyme that catalyzes this reaction is glutamate synthase; it is
also known as glutamate:oxoglutarate aminotransferase (GOGAT). A GS/GOGAT complex
exists in plants and allows them to cope with conditions of limited nitrogen
availability. Enzymes that catalyze transamination reactions require pyridoxal
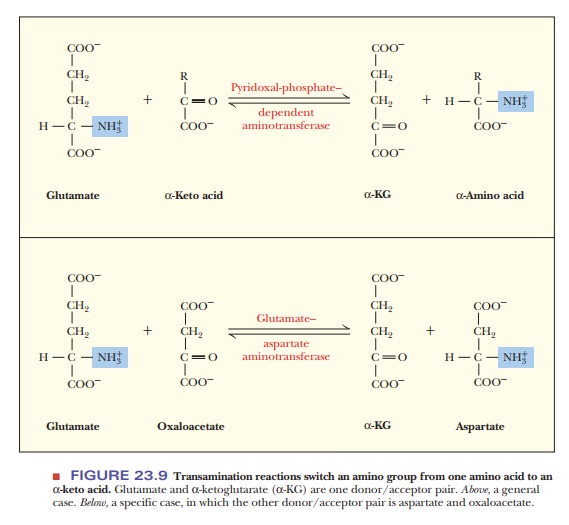
phosphate as a coenzyme (Figure 23.8).
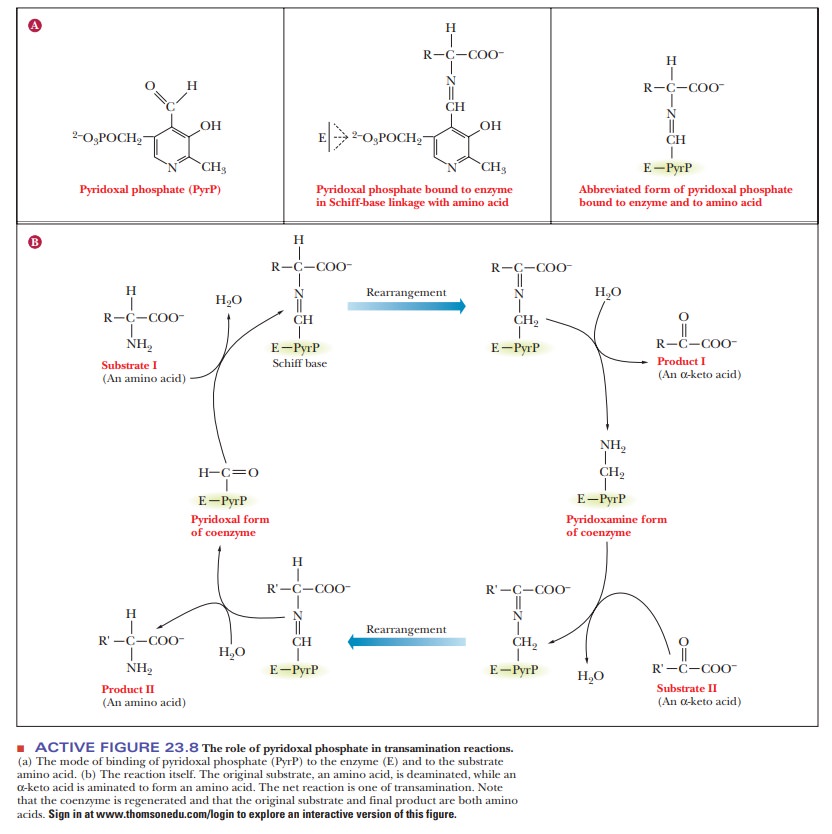
Pyridoxal phosphate (PyrP) forms a Schiff base with the amino group of Substrate I (the amino-group donor). The next stage is a rearrangement fol-lowed by hydrolysis, which removes Product I (the α-keto acid corresponding to Substrate I). The coenzyme now carries the amino group (pyridoxamine). Substrate II (another α-keto acid) then forms a Schiff base with pyridoxamine. Again there is a rearrangement followed by a hydrolysis, which gives rise to Product II (an amino acid) and regenerates pyridoxal phosphate. The net reac-tion is that an amino acid (Substrate I) reacts with anα-keto acid (Substrate II) to form an α-keto acid (Product I) and an amino acid (Product II).
The amino group
has been transferred from Substrate I to Substrate II, forming the amino acid,
Product II. The overall reaction can be seen for a general case and for a
specific case in Figure 23.9. When not involved with one of the substrates, the
pyridoxal group is bound in a Schiff-base linkage to an active site ε-NH2 group of lysine. Pyridoxal phosphate is a versatile
coenzyme that is also involved in other reactions, including decarboxylations,
racemizations, and movement of hydroxymethyl groups, as we shall see with the
conversion of serine to glycine.
What is the importance of one-carbon transfers?
In addition to transamination reactions, one-carbon transfer
reactions occur frequently in amino acid biosynthesis. A good example of a
one-carbon transfer can be found in the reactions that produce the amino acids
of the serine family. This family also includes glycine and cysteine. Serine
and glycine themselves are frequently precursors in other biosynthetic
pathways. A discussion of the synthesis of cysteine will give us some insight into the metabolism of
sulfur, as well as that of nitrogen.
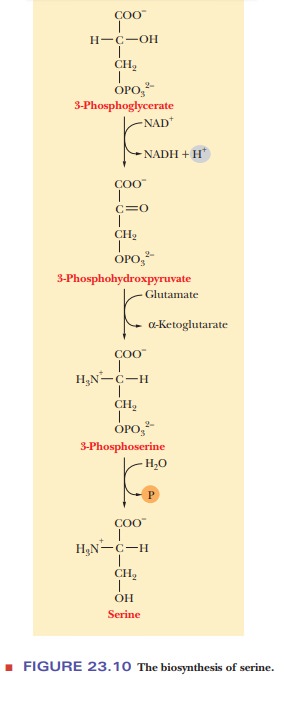
The
ultimate precursor of serine is 3-phosphoglycerate, which is obtainable from
the glycolytic pathway. The hydroxyl group on carbon 2 is oxidized to a keto
group, giving anα-keto acid. A transamination reaction in which
gluta-mate is the nitrogen donor produces 3-phosphoserine and α-ketoglutarate. Hydrolysis of the phosphate group then gives rise
to serine (Figure 23.10).
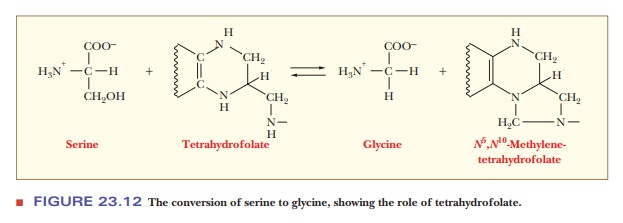
The
conversion of serine to glycine involves the transfer of a one-carbon unit from
serine to an acceptor. This reaction is catalyzed by serine hydroxymethylase, with pyridoxal phosphate as a coenzyme.
The acceptor in this reaction is tetra-hydrofolate,
a derivative of folic acid and a frequently encountered carrier
ofone-carbon units in metabolic pathways. Its structure has three parts: a
substi-tuted pteridine ring, p-aminobenzoic
acid, and glutamic acid (Figure 23.11). Folic acid is a vitamin that has been
identified as essential in preventing birth defects; consequently, it is now a
recommended supplement for all women of
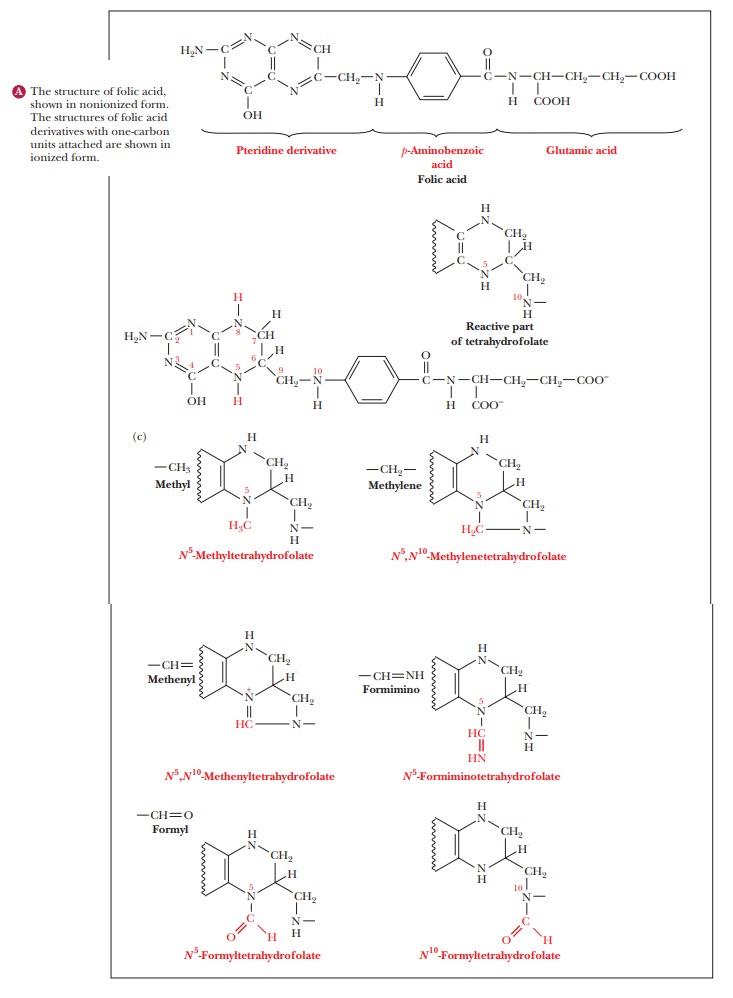
Serine + Tetrahydrofolate -> Glycine
+ Methylenetetrahydrofolate + H2O
The
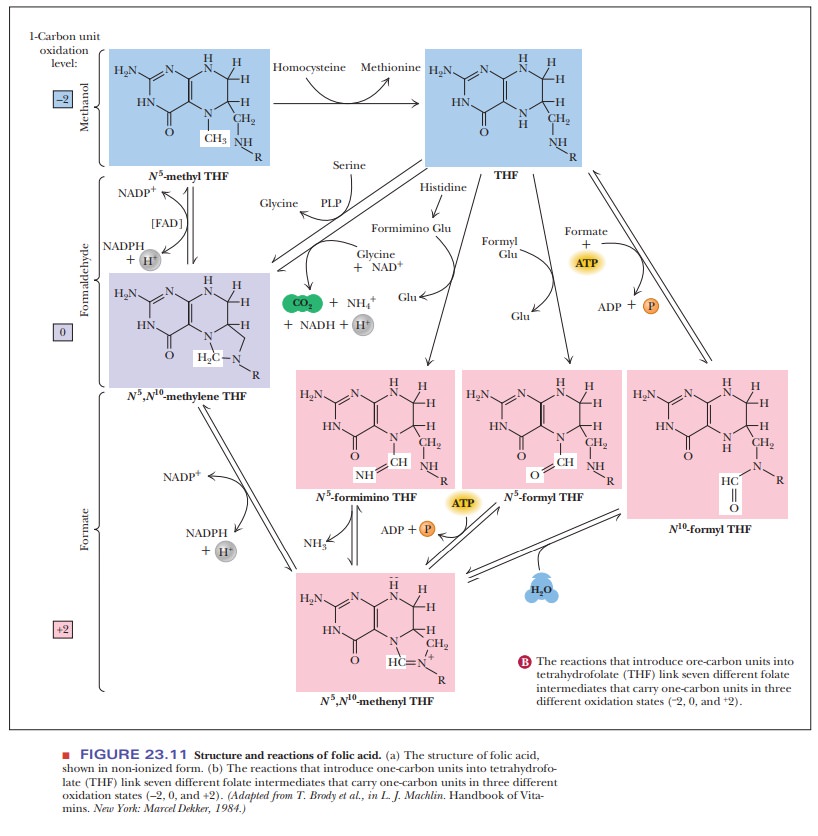
one-carbon unit transferred in this reaction is bound to tetrahydrofolate,
forming N5,N10-methylenetetrahydrofolate,
in which the methylene (one-carbon) unit is bound to two of the nitrogens of
the carrier (Figure 23.12). Tetrahydrofolate is not the only carrier of one-carbon
units. We have already encountered biotin, a carrier of CO2, and we
have discussed the role that biotin plays in gluconeogenesis and in the
anabolism of fatty acids.
The
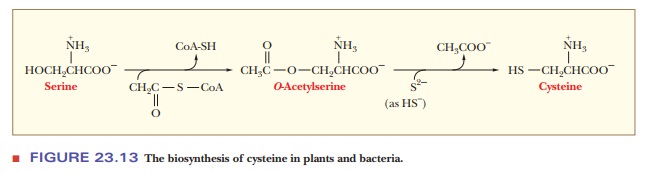
conversion of serine to cysteine involves some interesting reactions. The source
of the sulfur in animals differs from that in plants and bacteria. In plants
and bacteria, serine is acetylated to form O-acetylserine.
This reaction is cata-lyzed by serine
acyltransferase, with acetyl-CoA as the acyl donor (Figure 23.13).
Conversion of O-acetylserine to
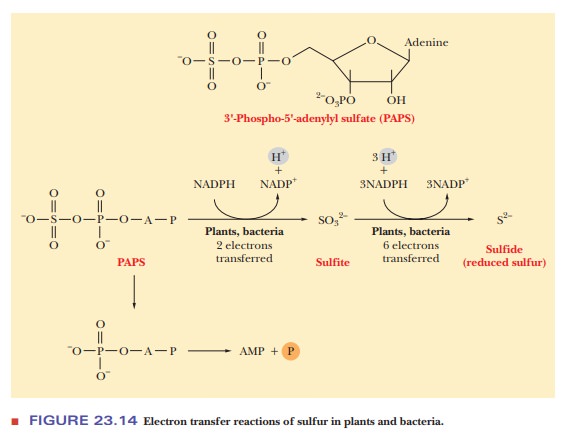
cysteine requires production of sulfide by a sulfur donor. The sulfur donor for
plants and bacteria is 3'-phospho-5'-adenylyl sulfate. The sulfate group is
reduced first to sulfite and then to sulfide (Figure 23.14). The sulfide, in
the conjugate acid form HS–, displaces the acetyl group of the O-acetylserine to produce cysteine.
Animals form cysteine from serine by a different pathway because they do not
have the enzymes to carry out the sulfate-to-sulfide conversion that we have
just seen. The reaction sequence in animals involves the amino acid methionine.
Methionine,
which is produced by reactions of the aspartate family (see the BiochemistryNow
Interactive website at www.thomsonedu.com/login)
in bacte-ria and plants, cannot be produced by animals. It must be obtained
from dietary sources. It is an essential
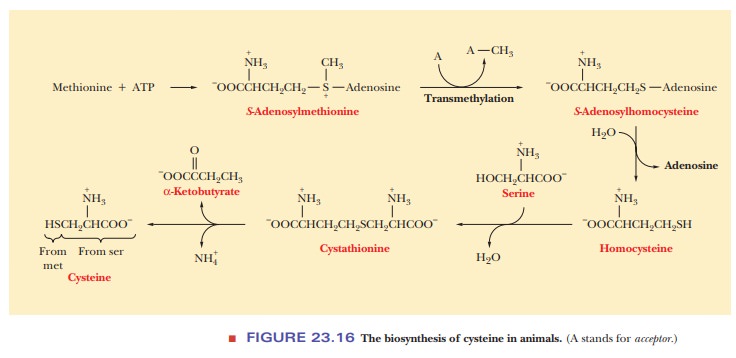
amino acid because it cannot be synthesized by the body. The ingested
methionine reacts with ATP to form S-adenosylmethionine(SAM), which has
a highly reactive methyl group (Figure 23.15). This compoundis a carrier of
methyl groups in many reactions. The methyl group from S-adenosylmethionine can be transferred to any one of a number of
acceptors, producing S-adenosylhomocysteine.
Hydrolysis of S-adenosylhomocysteine
in turn produces homocysteine. Cysteine can be synthesized from serine and
homocysteine, and this pathway for cysteine biosynthesis is the only one
available to animals (Figure 23.16). Serine and homocysteine react to produce
cystathio-nine, which hydrolyzes to form cysteine, NH4+,
and α-ketobutyrate.

It is
worth noting that we have now seen three important carriers of one-carbon
units: biotin, a carrier of CO2; tetrahydrofolate (FH4),
a carrier of methylene and formyl groups; and S-adenosylmethionine, a carrier of methyl groups.
Summary
Two of the most important classes of reactions in the biosynthesis
of amino acids are transamination reactions and one-carbon transfers.
The amino acids glutamate and glutamine are the principal donors of
amino groups in transamination reactions.
Carriers of one-carbon groups include biotin, S-adenosylmethionine, and derivatives of folic acid.
Related Topics