Chapter: Biochemistry: The Metabolism of Nitrogen
Nitrogen Fixation
Nitrogen Fixation
Bacteria
are responsible for the reduction of N2 to ammonia (NH3).
Typical nitrogen-fixing bacteria are symbiotic organisms that form nodules on
the roots of leguminous plants, such as beans and alfalfa. Many free-living
microbes and some cyanobacteria also fix nitrogen. Plants and animals cannot
carry out nitrogen fixation. This conversion of molecular nitrogen to ammonia
is the only source of nitrogen in the biosphere except for that provided by
nitrates. The conjugate acid form of NH3, ammonium ion (NH4+),
is the form of nitrogen that is used in the first stages of the synthesis of
organic compounds. Parenthetically, NH3 obtained by chemical
synthesis from nitrogen and hydrogen is the starting point for the production
of many synthetic fertilizers, which frequently contain nitrates.
How is nitrogen from the atmosphere incorporated into biologically useful compounds?
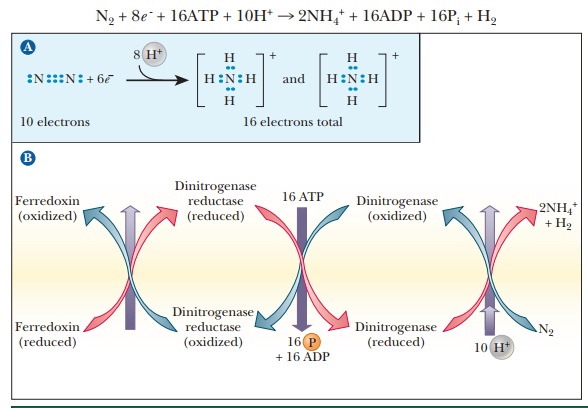
The nitrogenase enzyme complex found in nitrogen-fixing bacteria catalyzes the production of ammonia from molecular nitrogen. The half-reaction of reduction (Figure 23.2a) is in which six electrons are used to reduce molecular nitrogen to ammonium ion.
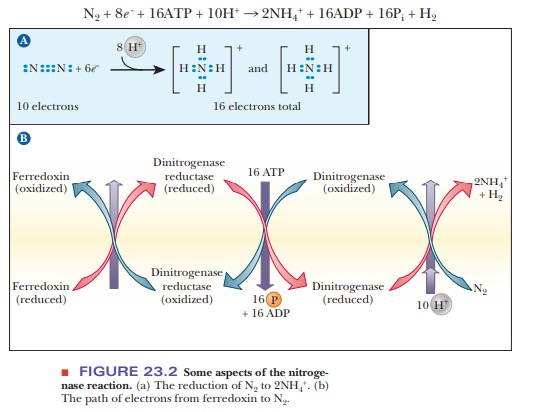
An
additional two electrons are used to reduce hydrogen ion to H2. The
total reaction catalyzed by nitrogenase is an eight-electron reduction.
The
half-reaction of oxidation varies because different organisms vary in terms of
the substance oxidized to supply electrons. Several proteins are included in
the nitrogenase complex. Ferredoxin is one of them (this protein also plays an
important role in electron transfer in photosynthesis;). There are also two proteins
specific to the nitrogenase reaction. One is an iron–sulfur (Fe–S) protein
called dinitrogenase reductase. The
other is an iron–molybdenum (Fe–Mo) protein, called dinitrogenase. The flow of
electrons is from ferredoxin to dinitrogenase reductase to dinitrogenase to
nitrogen (Figure 23.2b). The nature of the nitrogenase complex is a subject of
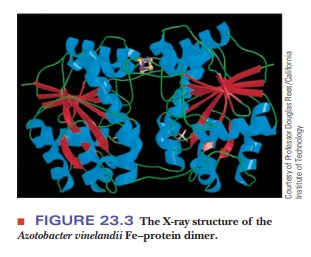
active research. Significant progress has been made in this work with the
deter-mination by X-ray crystallography of the three-dimensional structure of
both the Fe protein and the Fe–Mo protein from Azotobacter vinelandii (Figure 23.3). The Fe protein is a dimer
(“the iron butterfly”), with the iron–sulfur cluster located at the butterfly’s
head. The nitrogenase is even more complicated, with several types of subunits
arranged into tetramers. Ferredoxin, dinitrogenase reductase, and dinitrogenase
combine to perform a series of single-electron transfers, eventually
transferring the eight electrons necessary to complete the reduction of N2
to NH4+. It is worth noting that the reactions of
nitrogen fixa-tion consume a great deal of energy. It is estimated that about
half of the ATP produced from photosynthesis in legumes is used to fix
nitrogen.
Summary
Nitrogen enters the biosphere by the process of nitrogen fixation.
Atmospheric nitrogen is converted to ammonia in its conjugate acid form,
ammonium ion.
The nitrogenase enzyme found in root nodules of leguminous plants
cata-lyzes crucial reactions in nitrogen fixation.
Related Topics