Chapter: Biochemistry: The Metabolism of Nitrogen
Amino Acid Catabolism
Amino Acid Catabolism
When we specifically focus on the catabolism of amino acids, the first step we consider is the removal of nitrogen by transamination. Transamination reactions are also important in the anabolism of amino acids, so it is important to remind ourselves that anabolic and catabolic pathways are not the exact reverse of each other, nor do they involve exactly the same group of enzymes. In catabolism, the amino nitrogen of the original amino acid is transferred to α-ketoglutarate to produce glutamate, leaving behind the carbon skeletons. The fates of the carbon skeleton and of the nitrogen can be considered separately.
What is the fate of the carbon skeleton in amino acid breakdown?
Breakdown of the carbon skeletons of amino acids follows two
general pathways, the difference between the two pathways depending on the type
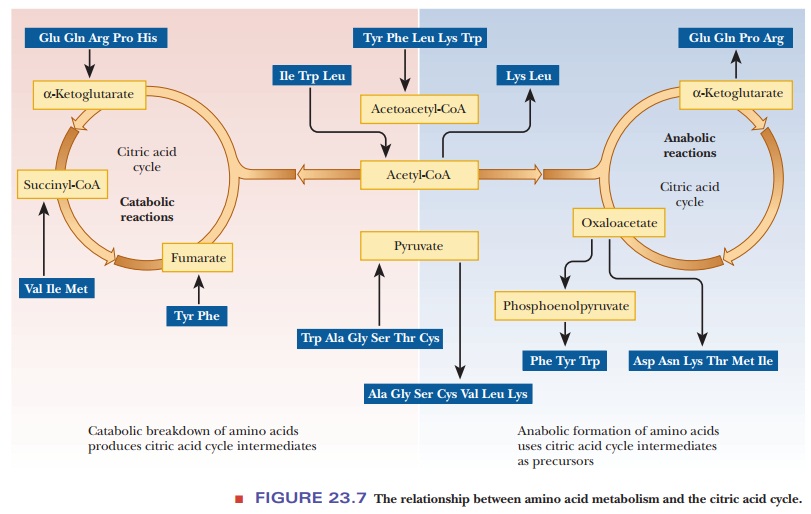
of end product. A glucogenic amino
acid yields pyruvate or oxaloacetate on degradation. Oxaloacetate is the
starting point for the production of glucose by gluconeogenesis. A ketogenic amino acid breaks down to
acetyl-CoA or acetoacetyl-CoA, leading to the formation of ketone bodies. The
carbon skeletons of the amino acids give rise to metabolic intermediates such
as pyruvate, acetyl-CoA, acetoacetyl-CoA, α-ketoglutarate, succinyl-CoA, fumarate, and
oxaloacetate (see Figure 23.7). Oxaloacetate is a key intermediate in the
breakdown of the carbon skeletons of amino acids because of its dual role in
the citric acid cycle and in gluconeogenesis. The amino acids degraded to
acetyl-CoA and acetoacetyl-CoA are used in the citric acid cycle, but mammals
cannot synthesize glucose from acetyl-CoA. This fact is the source of the
distinction between glucogenic and ketogenic amino acids. Glucogenic amino
acids can be converted to glucose, with oxaloacetate as an intermediate, but
ketogenic amino acids cannot be converted to glucose. Some amino acids have
more than one pathway for catabolism, which explains why four of the amino acids
are listed as both glucogenic and ketogenic.
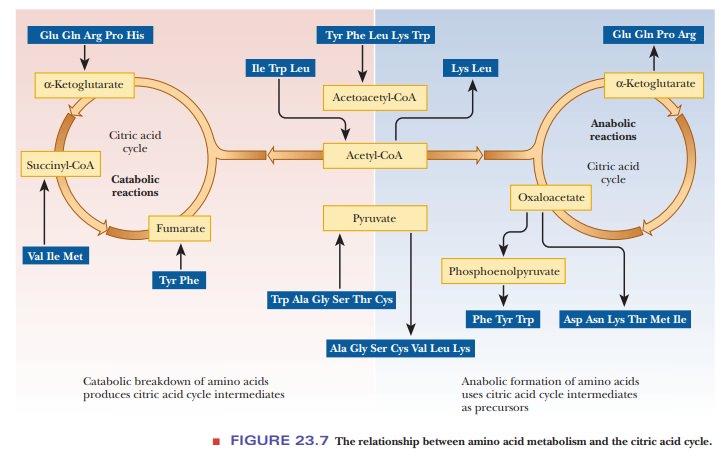
Excretion of Excess Nitrogen
The nitrogen portion of amino acids is involved in transamination
reactions in breakdown as well as in biosynthesis. Excess nitrogen is excreted
in one of three forms: ammonia (as
ammonium ion), urea, and uric acid (Figure 23.17).
Animals, such as fish, that live in an aquatic environment excrete
nitrogen as ammonia; they are protected from the toxic effects of high
concentrations of ammonia not only by the removal of ammonia from their bodies
but also by rapid dilution of the excreted ammonia by the water in the
environment. The principal waste product of nitrogen metabolism in terrestrial
animals is urea (a water-soluble compound); its reactions provide some
interesting comparisons with the citric acid cycle. Birds excrete nitrogen in
the form of uric acid, which is insoluble in water. They do not have to carry
the excess weight of water, which could hamper flight, to rid themselves of
waste products.
What is the role of the urea cycle in amino acid breakdown?
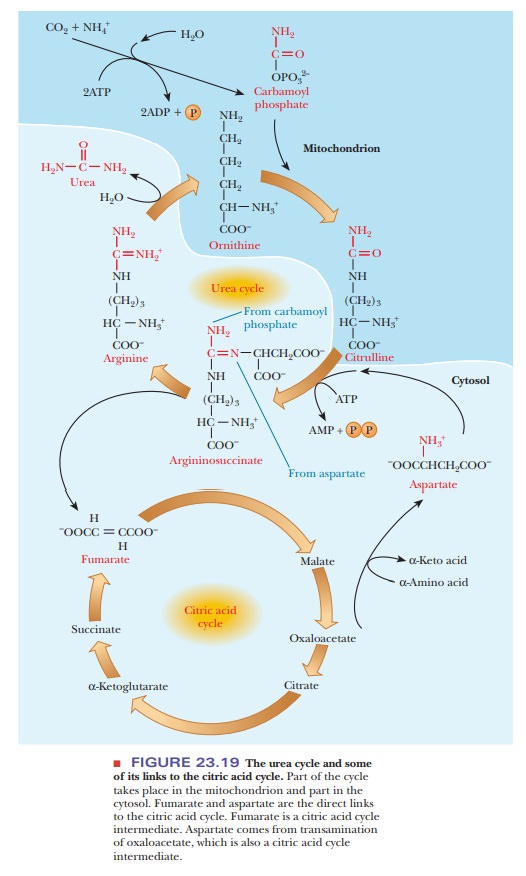
A central pathway in nitrogen metabolism is the urea cycle (Figure 23.18). The nitrogens that enter the urea cycle come from several sources. One of the nitrogens of urea is added in the mitochondria, and its immediate precursor is glutamate, which releases ammonia via glutamate dehydrogenase. However, the ammonia nitrogens of glutamate have ultimately come from many sources as a result of transamination reactions. Mitochondrial glutaminase also provides free ammonia that can enter the cycle.
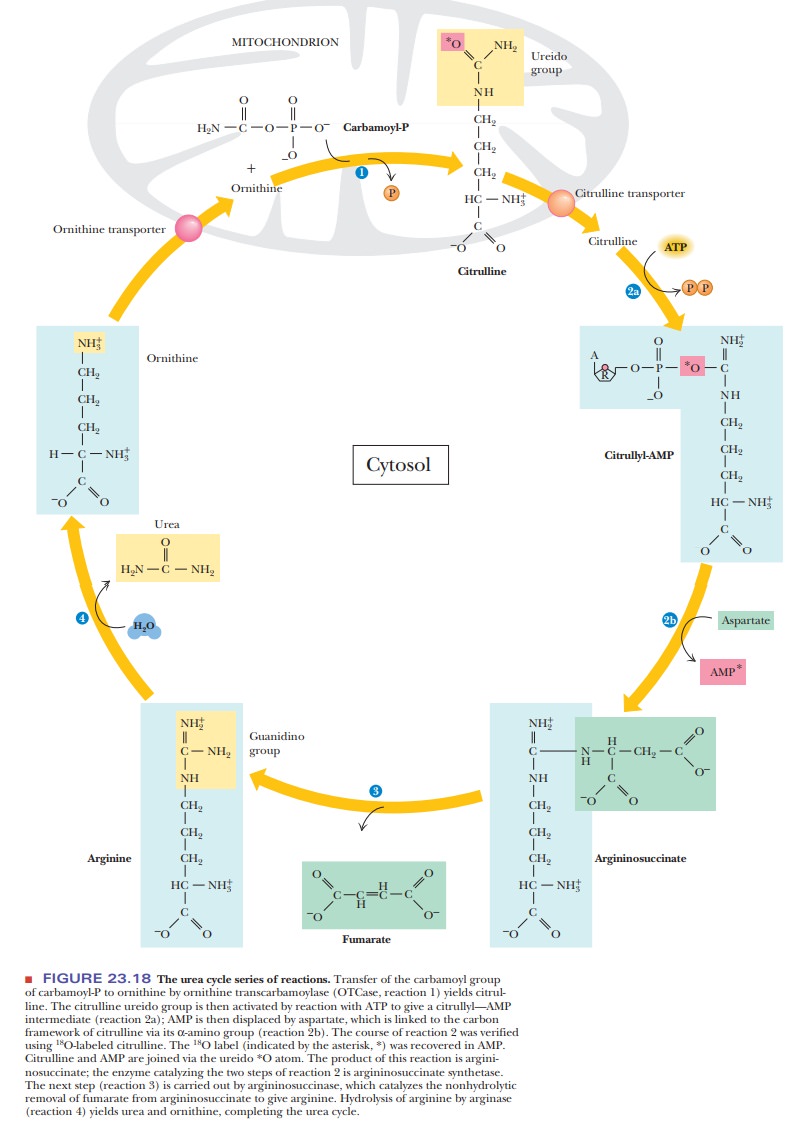
A condensation reaction between the ammonium
ion and carbon dioxide produces carbamoyl
phosphate in a reaction that requires the hydrolysis of two molecules of
ATP for each molecule of carbamoyl phosphate. Carbamoyl phosphate reacts with ornithine (Step to form citrulline. Citrulline is then
transported to the cytosol. A second nitrogen enters the urea cycle when
aspartate reacts with citrulline to form argininosuccinate
in another reaction that requires ATP (AMP and PPiareproduced in
this reaction; Step 2). The amino group of the aspartate is the source of the
second nitrogen in the urea that will be formed in this series of reactions.
Argininosuccinate is split to produce arginine
and fumarate (Step 3). Finally,
arginine is hydrolyzed to give urea and to regenerate ornithine, which is
transported back to the mitochondrion (Step 4). The biosynthesis of arginine
from ornithine is discussed on the Biochemistry Interactive website. Another
way of looking at the urea cycle is to consider arginine as the immediate
precursor of urea and to see it as producing ornithine in the process.
According to this point of view, the rest of the cycle is the regeneration of
arginine from ornithine.
The
synthesis of fumarate is a link between the urea cycle and the citric acid
cycle. Fumarate is, of course, an intermediate of the citric acid cycle, and it
can be converted to oxaloacetate. A transamination reaction can convert
oxa-loacetate to aspartate, providing another link between the two cycles
(Figure 23.19). In fact, both pathways were discovered by the same person, Hans
Krebs. Four high-energy phosphate bonds are required because of the production
of pyrophosphate in the conversion of aspartate to argininosuccinate.
In
humans, urea synthesis is used to excrete excess nitrogen, such as would be
found after consuming a high-protein meal. The pathway is confined to the
liver. Note that arginine, the immediate precursor to urea, is the most
nitrogen-rich amino acid, but the source of the nitrogen in the arginine
varies. The major control point is the mitochondrial enzyme carbamoyl-phosphate synthe-tase I (CPS-I),
and the formation of carbamoyl-phosphate is the committed stepin the urea
cycle. CPS-I is allosterically activated by N-acetylglutamate:
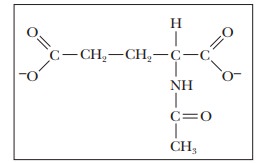
N-Acetylglutamate is formed by a reaction between glutamate and acetyl-CoA, which is catalyzed by N-acetylglutamate synthase. This enzyme is activated by increased concentrations of arginine. Thus, when amino acid catabolism is high, large amounts of glutamate are present from degradation of glutamine, from synthesis via glutamate dehydrogenase, and from transamination reac-tions. Increased glutamate levels lead to increased levels of N-acetylglutamate followed by increasing the activity of the urea cycle.
In addition, any time arginine
builds up, either because of protein catabolism or because ornithine is
building up because of a low level of CPS-I activity, the arginine stimulates
synthesis of N-acetylglutamate and
therefore increases the CPS-I activity.
Summary
The carbon skeleton has two fates in the breakdown process. Some
car-bon skeletons give rise to pyruvate or oxaloacetate, which can be used in
gluconeogenesis. Others give rise to acetyl-CoA or acetoacetyl-CoA, which can
form lipids.
The urea cycle, which has links to the citric acid cycle, plays a
central role in nitrogen metabolism. It is involved in both the anabolism and
the catabolism of amino acids.
Related Topics