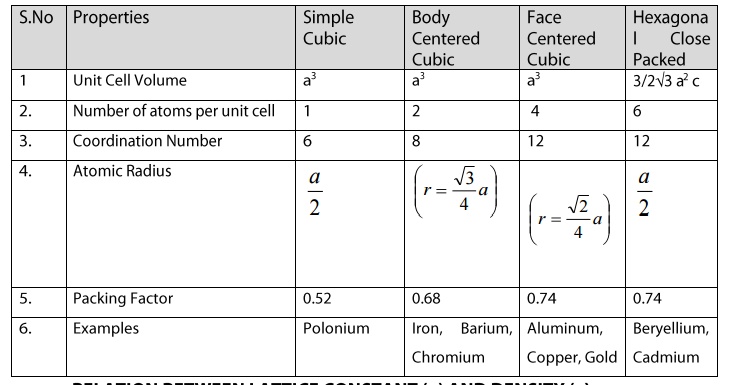
Chapter: Physics : Crystal Physics
Properties of some Crystal Structure - Sodium choride, Diamond cubic
Properties of some Crystal Strucure
RELATION BETWEEN LATTICE CONSTANT
(a) AND DENSITY(ρ)
Consider
a cubic crystal of lattice constant a
Density
of the crystal = ρ
Volume
of the unit cell = a3
Mass
of the unit cell = ρ a3
Density
= mass /volume
Number
of atoms per unit = n
Atomic
weight of the material = M
Avogadro’s
number =N
Avogadro’s
number is the number of atoms per kg mole of the substance.
Mass
of each atom = M/N
Mass
of the unit cell = mass of the each atom in unit cell x number of atoms per
unit cell
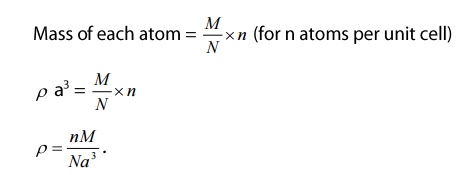
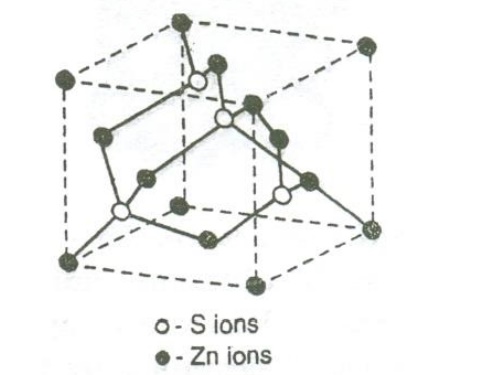
Diamond
cubic structure:
Diamond
has face centered structure (FCC) with the basis of two carbon atoms viz., ‘X’
and ‘Y’. The ‘X’ atom is located with an origin (0, 0, 0) and the Y atom is
located with an orgin of (a/4 , a/4, a/4) (i.e) one quarter of the way along
the body diagonal as shown in the figure
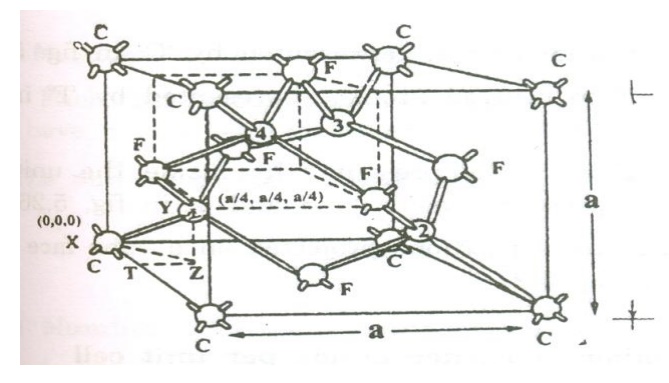
Thus
from the figure we can see that diamond structure is formed due to the
combination of two interpenetrating FCC sub lattices, having the origin (0,0,0)
and (1/4,1/4,1/4) along the body diagonal.
Let
us now discuss some of the important parameters of the diamond
1. Number of atoms per unit cell
i.
Corner atoms, represented by ‘F’ as
shown in the figure
ii.
Face centered atoms, represented by ‘F’
as shown in the figure.
iii.
Four atoms present inside the unit cell
an s represented as 1, 2.3,4 as shown in the figure.
iv.
The three type of atoms positions
projected on a cube face is as shown.
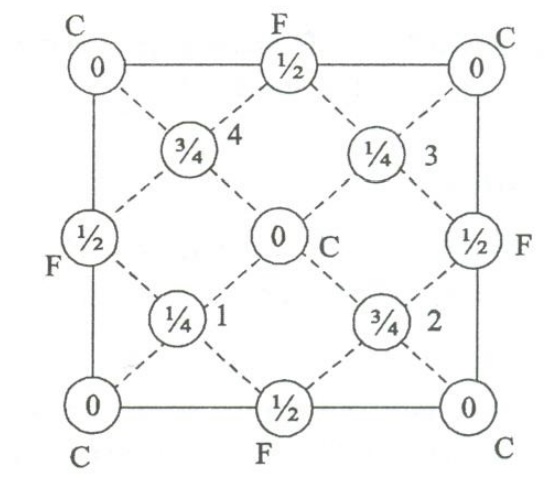
i.
Number of atoms per unit cell:
Each
corner atom is shared by 8 unit cells. Similarly we have 8 corner atoms in an
unit cell.
The
number of corner atoms in an unit cell.
ii.
Number of face centered atoms per unit
cell
Each
face centered atom is shared by 2 unit cells. Similarly, we have 6 face centered atoms.
The
number of face centered atom per unit cell
= ½ x 6 = 3 atoms
iii. Number of atoms inside the unit cell
Inside
the unit cell we have 4 atoms, represented by 1,2,3,4 in the figure which is
shared by that particular unit cell alone.
Total number of atoms per unit cell = 1+3+4=8
2.
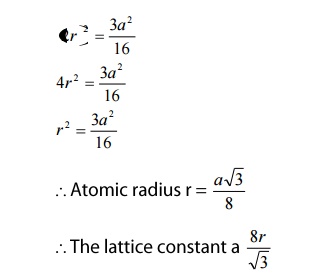
Atomic radius
We know atomic radius is defined as half
the distance between any two nearest neighbor atoms, which have direct contact
with each other.
Here, the corner atoms do not have contact
with each other and the face centered atoms also do not have contact with the
corner atoms. But both the face centered
atoms and the corner atoms have contact with the 4 atoms (1,2,3,4)
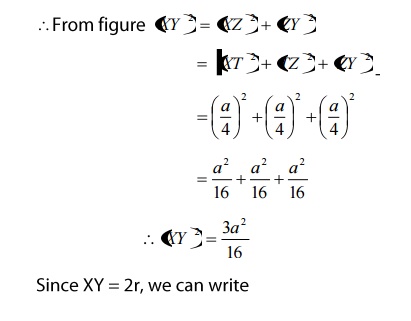
From figure, we can see that the nearest
two neighbors which have direct contact (shown by double line) are atoms'X'
and'Y'.
Let
us draw perpendicular to the Y atom, which meets the unit cell at a point'Z' as
shown in the figure, which is a distance of a/4.
3.
Coordination number:
We
know that the coordination number is the nearest atoms (shown by double line) for
Y atom is 4. Therefore the coordination number of diamond is 4
4. Atomic packing factor:
we
know
Atomic
packing factor(APF)=
volume
occupied by the atoms per unit cell (v)/volume of the unit cell(V) --- > (1)
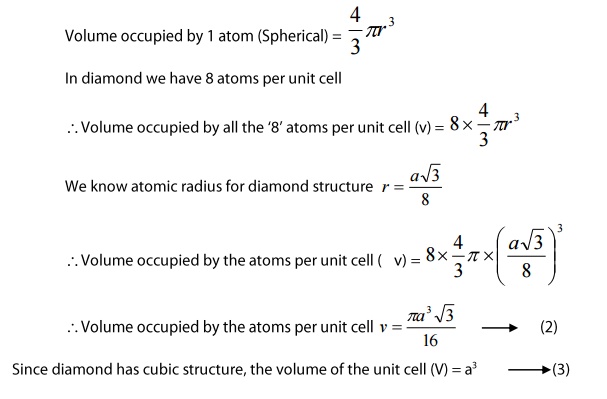
Substituting
equations(2) and (3) in (1) we get
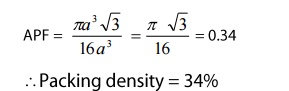
Thus
we can say that 34% voulme of the unit cel in diamond cubic structure is
occupied by the atoms and the remaining 66% volume is vacant.
Since
the packing density is very low, it is termed as very loosely packed structure.
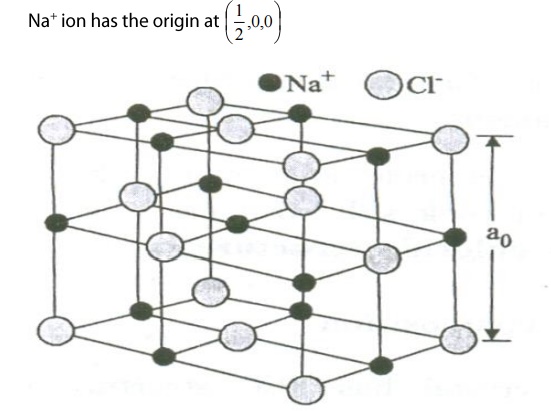
SODIUM CHLORIED
STRUCTURE:
Ionic
solids are made up of cat ion(+ion) and anions (-ion). Genrally, the cations (+
ve) are smaller in size to anions.
Sodium choride and many other ionic crystals
crystalline in rock slat structure which is also known as sodium chloride
structure.
Structure
compoition:
NaCl
crystal has FCC structure with Na+ ion and cl ion as shown in the figure.
The
Cl- ions are situated at the corners as well as at the centers of
the faces of the cube. Na+
ions are situated exactly at the middle of the axial length of the unit cell
along each axis.
Thus NaCl crystal can be thought of
as a compound of two FCC Na+ and
Cl
sub lattices
When one of the Cl ions has the origin
at (0,0,0) then one of the nearest
Na+
ion has origin at (1/2,0,0)
Let
us discuss the important parameters of the NaCl crystal.
In NaCl structure, it has two types of
ions namely, Na+ and Cl- . Let us find the number of
sodium ions separately.
a.
Number of Na+ ions per unit cell
Na+
ion is located at the midpoint of the axial length. There are 12 such mid
points Na+ ions. Each sodium ion is shared by 4 adfacent unit cell.
Share
of one unit cell
Number
of mid point Na+ ions per unit cell
¼
x 12 = 3 ions.
One
sodium ion is located at the center of the unit cell.
Therefore,
the number of sodium ions per unit cell = 3+1=4 ions
b.
Number of Cl- ions per unit cell
Here,
there are two types of Cl- ion namely Corner Cl ion and face
centered Cl ion as shown in the figure.
There
are 8 chlorine atoms in the corners and they are shared by 8 adjacent unit cells.
Number
of corner Cl- ions per unit
cell
=
1/8 x 8 1 ion.
Each
face centered Cl- ion is shared by 2 adjoining unit cell. There are
6 face centered Cl- ions.
Number
of face centered Cl- ions per unit cell
=
½ x 6 = 3 ions.
Therefore
total number of chlorine ions per unit cell = 1+3 = 4 ions.
Thus,
there are 4 Na+ and 4Cl- ions per unit cell. That is total number of sodium and
chlorine ions per unit cell are 8.
2. Coordination number:
Each
Na+ and 6 Cl- ions as nearest neighbors and similarly each Cl- ion has 6 Na+
ions has nearest neighbours. Hence, the coordination number of NaCl for opposite
kind of ions is 6.
3. Nearest neighbours distance
The
nearest neighbor distance is a/2
Related Topics