Chapter: Physics : Crystal Physics
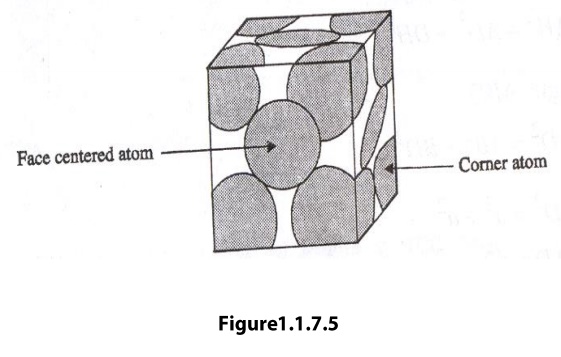
Face centered cubic (FCC) Structure
Face Centered Cubic
A
face centered cubic cell consists of eight corner atoms and six face centered
atoms. The arrangement of atoms in face centered cubic unit is as shown in
figure.
(i)
Number
of atoms per unit cell
An
FCC unit cell consists of eight corner atoms and each and every corner atoms is
shared by eight adjacent unit cell. Therefore, each and every corner atom
contributes 1/8 of its part to one unit cell. The total number of atoms by
corner atom = 1/8 x 8 = 1 atom.
In
addition, there are 6 atoms at the face centers of the cube. Each face-centered
atom is shared by two surround unit cells. Hence, the
number of face centered atoms in unit cell,
=1/2
x 6 =3 atoms.
Therefore,
total number of atoms in one unit cell = 1 + 3 = 4 atoms.
(ii)
Coordination
Number
In
this case, there are eight atoms at the eight corners of the unit cell and 6
atom at the center of the six faces.
For
any corner atom of the unit cell, the nearest atom is face-centered atom. For
any corner atom, there are four face-centered atoms in its plane and four above
its plane and four below its plane.
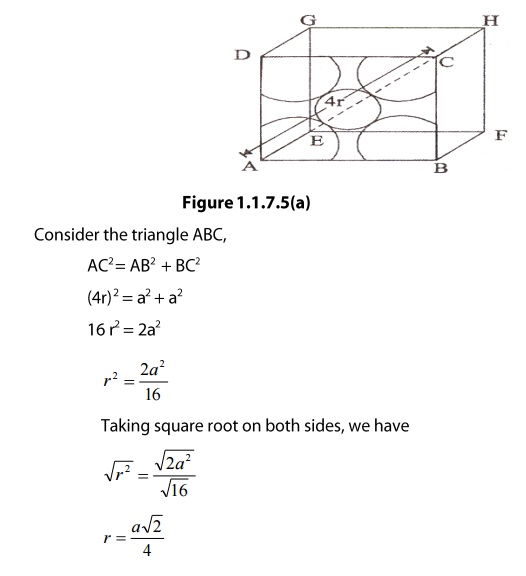
(iii)
Atomic
Radius
For
a body centered cubic unit cell, the atomic radius can be calculated from
figure as follows.
(iv)
Packing
Factor
The
number of atoms present in an FCC unit cell is four. Therefore, the packing
factor of the FCC unit cell be written as
Number
of atoms per unit cell = 4
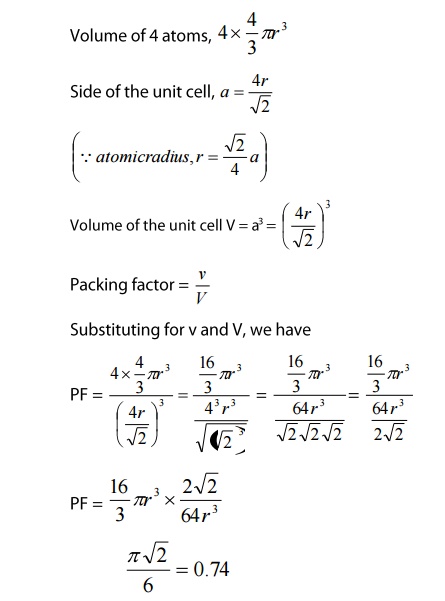
This
shows that 74% of the volume of an FCC unit cell is occupied by atoms and the
remaining 26% volume of the unit cell is Vacant.
Thus
the packing density is 74%.
Since
the packing density is very high, the FCC structure has closely (or) tightly
packed structure.
Related Topics