Chapter: Physics : Crystal Physics
Body centered cubic(BCC) Structure
Body centered cubic (BCC) Structure
In
body centered cubic structure, the unit cell has one atom at each corner of the
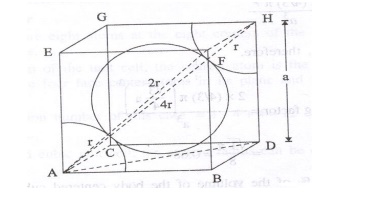
cube and one at body center of the cube. Figure 3.8 shows the arrangement of
the atoms in a bcc cell.
(i)
Number
of atoms per unit cell
In
a body centered crystal structure, the atoms touch along the diagonal of the
body. Each and every corner atoms are shared by eight adjacent unit cells.
Therefore, the total number of atoms contributed by the corner atoms is 1/8 x 8
=1 atom.
One
full atom at the center of the unit cell = 1 atom
Therefore,
total number of atoms present in the bcc unit cell = 1+1 = 2 atoms.
(ii)
Coordination
Number
The
coordination number of the body centered cubic unit cell is calculated as
follows.
Let
us consider a body-centered atom. The nearest neighbor for a bcc atom is corner
atom. A body centered atom is surrounded by 8 corner atoms. Therefore, the
coordination number of a bcc unit cell is 8.
Atomic radius
For
a body centered unit cell, the atomic radius can be calculated as follows from
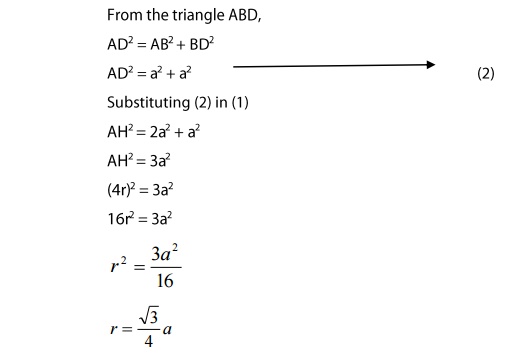
figure as follows. From figure, AH = 4r and DH = a
From
the triangle AHD,
AH2=AD2+DH2 ----> (1)
(iii)
Packing
Factor
The
number of atoms present in a unit cell = 2 atoms
Packing
Factor = (Number of atoms present per unit cell x Volume of atom) / Volume of
the Unit Cell
Number
of atoms per unit cell=2
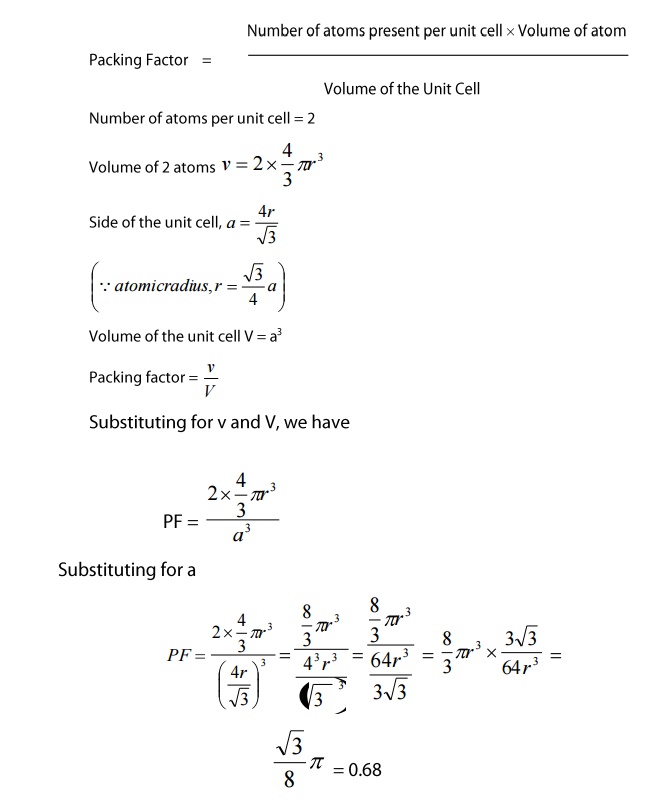
Therefore,
we can say that 68% volume of the unit cell of BCC is occupied by atoms and
remaining 32% volume is vacant.
Thus
the Packing Density is 68%.
Since
the packing density is greater than simple than cubic, it has tightly packed
structure, when compared to SC.
Related Topics