Chapter: Pharmaceutical Drug Analysis: Thin Layer Chromatography (TLC)
Preparation of Thin Layers on Plates - Thin Layer Chromatography (TLC)
PREPARATION OF THIN LAYERS ON PLATES
The paramount importance with regard to the preparation
of thin layer is that it must be uniform and consistent throughout. Various
means have been put forward to apply thin layers of powdered or their
suspen-sions or their slurries to the carrier plates with a view to achieve an
uniform layer throughout the length of the plates. These are namely :
(a) Pouring of Layers :
In order to obtain
layers of equal thickness, a measured amount of the suspen-sion or slurry is
placed on a given-size plate that is rested on an absolutely labelled surface.
The plate is subsequently tipped backward and forward to permit the slurry (or
suspension) to spread uniformly on the surface of the plate.
(b) Dipping :
Peifer* in 1962, was pioneer
in introducing this technique, whereby two plates at a time back-to-back are
dipped together in a slurry of the adsorbent in either chloroform or
chloroform-methanol. However, this particular methods is not much in use
now-a-days.
(c) Spraying :
Reitsema** was first to
develop this method by making use of a small paint-sprayer for the distribution
of the suspension or slurry onto the surface of the glass-plate.
Disadvantages : There are mainly two major disadvantages of this technique,
namely :
(i)
Non-uniformity of layers on a single-plate, and
(ii) Variation
observed from one plate to the other was significant.
Belgian Patent No : 625012 : It essentially consists of
spraying either molten or partially molten
absorbent onto a glass plate, for instance : an alumina film prepared by
melting and aluminium rod with an oxyacetylene flame and subsequently spraying
the molten adsorbent onto a glass plate.
(d) Spreading :
In this particular case,
the suspension or slurry is put in an ‘applicator’, which is subsequently moved
either over the stationary glass-plate or vice-versa
i.e., it is held stationary while the glass plate is pulled or pushed
through. This technique termed as ‘spreading’ usually yields uniform thin
layers on the glass plates.
Kirchner’s* technique essentially consists of :
·
selecting uniform surfaced glass plates,
·
placing them between glass or metal guides which are
thicker than glass plates by the amount that is desired for the adsorbent
layer, and
·
spreading the slurry on the glass plate with the help of
a glass rod.
Egon Stahl’s apparatus exclusively designed for the
application of thin-adsorbent layers which broadly comprises of two major parts, namely :
(i) Aligning Tray : It is a tray on which
the glass plates are placed in a series or in-a-line, and
(ii) Spreader : It holds the spreading
mixture (as a slurry or suspension) and applies it uniformly in a thin-layer.
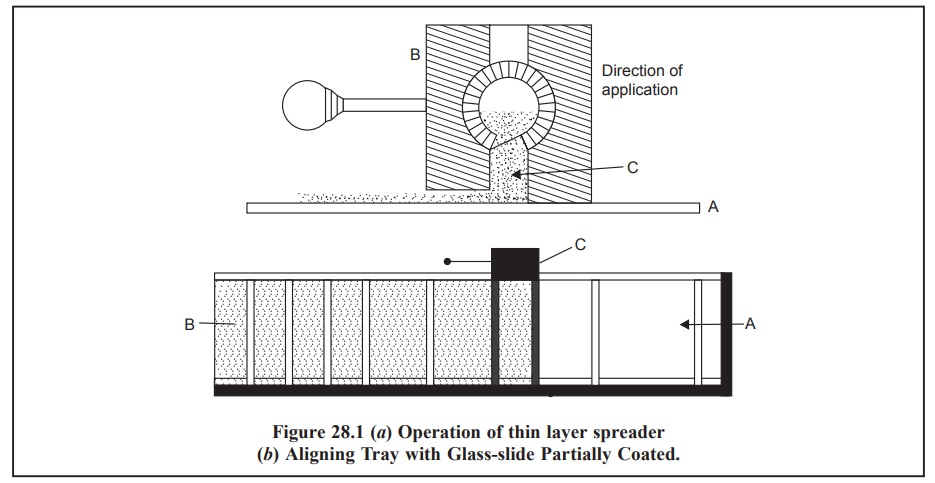
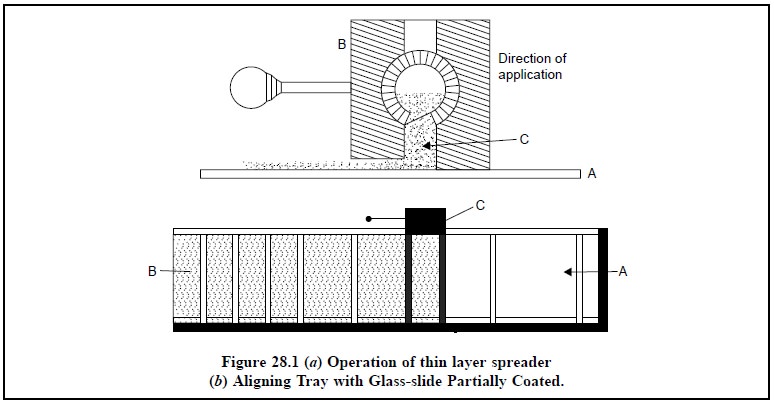
Figure 28.1 (a)
shows the operation of thin-layer spreader ; while Figure 28.1 (b) depicts the aligning tray with
glass-slide partially coated.
In Figure 28.1 (a)
A = Glass plate,
B = Spreader, and
C = Slurry of the adsorbent.
Here, the slurry (C) is put in the spreader (B) and then
moved along the direction of application onto the surface of the glass plate
(A) to obtain an uniform layer,
In Figure 28.1 (b)
A = Glass plate,
B = Layer of adsorbent, and
C = Aligning tray
The slurry of the adsorbent is introduced into the
aligning tray (C) which is then moved onto the glass plate (A) to obtain an
uniform layer of adsorbent (B).
In 1962, a well known West German firm DESAGA introduced
a much improved and simplified ver-sion of a TLC-spreader that could
conveniently give uniform layer thickness raging from 0.00 to 2,0 mm.
(e) ‘TLC-Plates ready-for Use’ (or Pre-coated
Plates)
E. Merck AG (Darmstadt, West Germany) was pioneer in
introducing two types of
ready-for-use TLC plates either on glass or polysheets (ethyleneterephthalate),
namely :
(i)
Ready-for-use TLC Plates with Cellulose-F, and
(ii)
Ready-for-use TLC Plates with Silica Gel F-254.
Interestingly, these precoated TLC-plates essentially
have : first, a special abrasive-resistant layer con-taining no gypsum ; and
secondly, the layer contains a reliable fluorescent indicator that is excited
to emit a fluorescence under either a short-wave or a long-wave UV light.
Advantages : These ‘ready-for-use’ TLC-plates have the following advantages namely :
·
It may be safely activated at 110-120° C, before, use,
·
The properties of the layer minimise spot-diffusion that
helps both more strong concentration of spots and more distinctive separations
with higher sensitivity,
·
It may accept more corrosive spray-reagents, for example
: conc. sulphuric acid, phosphoric acid, phosphomolybdic acid, perchloric acid
on antimony trichloride ; and the sprayed plates could be heated upto 110-120
°C without any darkening whatsoever,
·
The migration rate is slightly enhanced when compared to
hand coated plates, and
·
The TLC plates may be cut into strips by the aid of a
glass cutter applied on the reverse side.
Related Topics