Chapter: Pharmaceutical Drug Analysis: Thin Layer Chromatography (TLC)
Evaluation of the Chromatogram - Thin Layer Chromatography (TLC)
EVALUATION OF THE CHROMATOGRAM
After completing the detection procedure the various
separated solutes on the TLC plate are marked with the help of a sharp needle (e.g., pithing needle) ; subsequently,
their evaluation may be carried out either qualitatively or quantitatively, as
stated below :
1. Qualitative Evaluation
The Rf value (Retention Factor) various separated solutes
is determined accurately. The Rf value repre-sents the differences in rate of
movement of the components duly caused by their various partition coefficients i.e., their different solubility in the
mobile and stationary phases. In order words, the Rf value (relate to front) is-‘the ratio between the distance
starting point-centre of spot and distance starting point-solvent front’, thus
it may be expressed as :
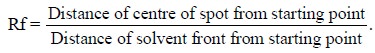
Important Points :
(i) Due to the
always longer path of the solvent front, the Rf value is invariably lesser than 1.
(ii) Rf value
is always constant for each component only under identical experimental
parameters, and
(iii) Rf value
depends upon a number of governing factors, such as : quality of the layer
material ; activation grade of the layer ; thickness of layer ; quality of
solvent ; equilibration of chamber ; chromatographic technique employed (e.g., ascending, descending) ; presence
of impurities ; and conc. of simple applied ; and
(iv) All possible anomalies in (iii) above may be eliminated by performing a co-chromatogram of a
standard substance along with that of a sample. Thus, the distance traversed by
a substance is compared with that of the standard (or reference). This ‘new’
relation is usually designated as Rst-value. Therefore, in short, it is
expressed as follows :
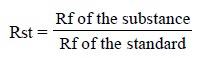
Unlike the Rf value, the Rst value may be more than 1.00
because here the substance under investiga-tion (i.e., sample) usually travels further than the standard.
In TLC, the qualitative evaluation is solely based on the
determination of Rf values of unknown spots vis-a-vis
Rf values of standard substances preferably on the same TLC plate so as to
avoid any possible error whatsoever.
2. Quantitative Analysis
The quantitative analysis of chromatographically
separated constituents may be carried out with high degree of accuracy and
precision in two manners, namely :
(i) Direct Method : i.e., the quantitative determinations is performed directly on the
adsorbent layer, and
(ii) Indirect Method : i.e., the separated constituents are quantitatively removed from,
the adsorbent and subsequently estimated after elution.
2.1. Direct Methods
The various methods under this category are, namely :
(i) Measurement of Spot-areas : This method
is solely based on a mathematical relationship existing between the prevailing
spot area and the amount of component present. It is not quite accurate due to
high random errors.
(ii) Densitometry : The intensity of the
colour of a component is measured on the chromatogram using a densitometer.
(iii) Spectrophotometry : Characterization of
the separated spots by reading the absorption or fluores-cence curves directly
from TLC plates is carried out with the help of Chromatogram Spectrophotometer
devised by Zeiss, Stahl and Jork.
Besides, IR-spectroscopy, reflectance spectroscopy, spark
chamber method etc., may also be employed for the direct evaluation of
chromatograms.
2.2. Indirect Methods
These methods are based on elution techniques, followed
by micro-analysis of the resultant eluate by adopting one or more of the
undermentioned known methods, namely :
Colorimetry ; Fluorimetry ; Radiometry ; Flame-photometry
; UV-Spectrophotometry ; Gravimetry ; Polarography ; Vapourphase Chromatography
;
Related Topics