Chapter: Pharmaceutical Drug Analysis: Thin Layer Chromatography (TLC)
Applications of TLC in Pharmaceutical Analysis
APPLICATIONS OF TLC IN PHARMACEUTICAL ANALYSIS
The technique of thin-layer chromatography (TLC) has been
used extensively in the domain of pharma-ceutical analysis for a variety of
specific and useful applications, for example :
(i) To identify
the presence of undesirable specific organic compounds present as impurities in
a number of pharmaceutical substances, namely : morphine in apomorphine
hydrochloride ; hydrazine in carbidopa ; 3-aminopropanol in dexampanthenol ;
etc.,
(ii) Related
substances present in official drugs, namely : related substances present in a
wide number of potent pharmaceutical substances e.g., aminophylline ; baclofen ; chloramphenicol ; carbamazepine
etc.,
(iii) Foreign
alkaloids present in alkaloidal drugs, for instance : atropine sulphate ;
codeine ;
(iv) Foreign
steroids present in steroidal drugs, for example : betamethasone valerate ;
(v) Ninhydrin
positive substances in official amino acids e.g.,
glutamic acid ; leucine ;
The various applications of TLC as cited above would be
discussed in the sections that follow :
1. PRESENCE OF SPECIFIC SUBSTANCES AS IMPURITIES IN DRUG SUBSTANCES
Examples : (1) Morphine in Apomorphine
Hydrochloride
Materials Required : Silica gel-G ; Mixture of
Acetonitrile : Dichloromethane : Ethyl acetate ; Anhydrous formic acid : Water (30 : 30 : 30 : 5 : 5) ; solution
(1) : 0.20% w/v of apomorphine in methanol ; solution (2) : 0.004% w/v of
apomorphine HCl in methanol ; (3) Morphine : 2% w/v in methanol ; sodium
nitrite solution (3% w/v in DW) ;
Procedure : Prepare the chromatogrphic
tank by lining the walls with sheets of filter paper ; pour the mobile-phase into the tank, saturating
the filter paper in the process, to a depth of 5 to 10 mm, close the tank and
allow it to stand at 20° to 25 °C for 1 hour for equilibration of the
mobile-phase in the chromatank. Apply separately to the TLC plate 5 μ l of each of two solutions (1)
and (2) of apomorphine hydrochloride and (3) of morphine in the form of
circular spots about 2 to 6 mm in diameter, and 15 to 20 mm from one end of the
plate and not nearer than 10 mm to the sides ; the two spots must be at least
10 mm apart. Mark the sides of the plate 15 cm from the line of application.
Allow the solvent to evaporate and place in the chromatank, ensuring that it is
nearly vertical as possible and that the spots are above the level of the
mobile-phase. Close the tank and allow to stand at 20° to 25°, unless the
mobile-phase has ascended to the marked lines. Remove the plate and dry it in a
current of cold air until all traces of solvent has disappeared and spray with
a solution of sodium nitrite. Expose the plate to ammonia vapour for a few
minutes and allow to stand in daylight for about 1 hour.
Observations : In the chromatogram obtained
with solution (1), there is no reddish orange spot with an Rf value of 0.3 to .5 relative to the principal spot (about 2% of
morphine). The test in not valid unless there is a clearly visible spot in the
chromatogram obtained with solution (2).
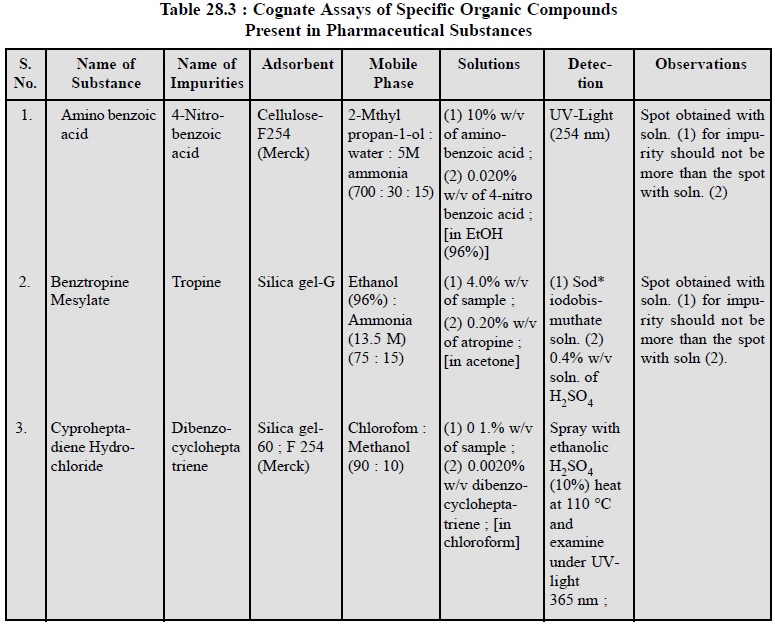
1.1. Cognate Assays
A number of other typical examples of pharmaceutical
substances containing specific organic com-pounds, that may be identified by
adopting the similar TLC technique are stated in Table 28.3 :
2. RELATED SUBSTANCES PRESENT IN OFFICIAL DRUGS
(1) Aminophylline :
Presence of Related Substances
Materials Required : Silica gel-G F254 ;
Mobile-phase (butan-1-ol : acetone : chloroform : 13.5 ammonia : : 40 : 30 : 30
: 10) : 100 ml ; Solution-1 : dissolve 0.2 g of sample in 2 ml of DW, warm and
dilute to 10 ml with methanol ; Solution (2) : dilute 1 vol. of soln. 1 to 200
vols. with methanol ;
Procedure : Apply separately to the coated
plate of silica get GF254 10 μ l each of solution (1) and (2). Follow the procedure as detailed previously, using the above
mobile phase. After removal of the plate, allow it to dry in air and examine
under UV-light (254 nm).
Observations : Any secondary spot in the
chromatogram obtained with solution (1) is not more intense than the spot in the chromatogram obtained with solution
(2).
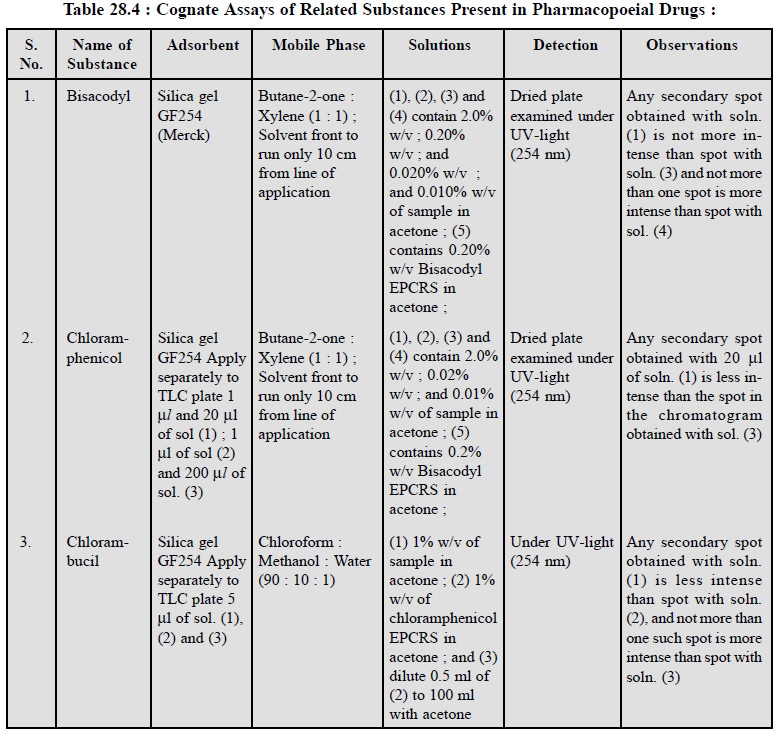
2.1. Cognate Assays
A good number of pharmaceutical substances do contain
‘related substances’ which can be identified by TLC methods as summarized in
Table 28.4 below :
3. FOREIGN ALKALOIDS PRESENT IN ALKALOIDAL DRUGS
Examples :
(1) Atropine Sulphate :
Foreign Alkaloids and Development Products :
Materials Required : Silica gel G ; mobile-phase
(acetone : water : 13, 5 M ammonia : : 90 : 7 : 3) : 100 ml ; solution (1, 2%
w/v of sample in methanol ; solution (2) : 0.02% w/v of sample in methanol ;
solution (3 : 0.01% w/v of
sample in methanol ; dilute potassium iodobismuthate
solution (dissolve 100 g of (+) –
tartaric acid in 500 ml of water and add 50 ml of
potassium iodobismuthate solution RI*) : 100 ml ;
Procedure : Apply separately to the coated
TLC plate 1 μ l of each of three solutions (1), (2) and (3). Develop the plate in the above
mobile-phase such that the solvent front is allowed to ascend only 10 cm above
the line of application. After removal of the plate, dry it at 100 °C to 105 °C
for 15 minutes, allow to cool and spray with dilute potassium iodobismuthate
solution until spots appear.
Observations : Any secondary spot in the
chromatogram obtained with solution (1) is not more intense than the spot obtained with solution (2), and not more
than one such spot is more intense than the spot obtained with solution (3).
3.1. Cognate Assay
The presence of ‘foreign
alkaloids’ in Codeine (BP)** may be determined by more or less an identical
method as already discussed earlier.
4. FOREIGN STEROIDS PRESENT IN STEROIDAL DRUGS
Example :
(1) Betamethasone
Valerate : Related Foreign Steroids :
Materials Required : Silica gel G ; mobile-phase :
(1, 2-dichloroethane : methanol : water : : 95 : 5 : 0.2) : 100 ml ; mixture of chloroform and methanol (9 : 1) : 50 ml
; solution (1) : betamethasone valerate sample : 1. 5% w/v ; solution (2) :
betamethasone valerate BPCRS/EPCRS*** : 1.5% w/v ; solution (3) : a solution
containing 0.030% w/v each of betamethasone EPCRS and betamethasone 21-valerate
BPCRS ; alka-line tetrazolium blue solution**** q.s. ;
Procedure : Apply separately to the coated
TLC plate 1 μ l of each of three solutions (1), (2) and (3) prepared in a mixture of
chloroform/methanol stated above. After removal of the plate, allow it to cool
dry in air until the solvents have evaporated, heat at 105 °C for 10 minutes,
cool and spray with alkaline tetrazolium blue solution.
Observations : (1) The principal spot in the
chromatogram obtained with soln. (1) corresponds in position, colour and intensity to that obtained with soln. (2),
Any secondary spot in the chromatogram obtained with
soln. (1) is not more intense than the proximate spot in the chromatogram, with
soln. (3).
5. NINHYDRIN POSITIVE SUBSTANCES PRESENT IN OFFICIAL AMINO ACIDS
Example :
1. Glutamic Acid
Materials Required : Silica gel-G ; mobile-phase
(glacial acetic acid : water : butan-1-ol : : 20 : 20 ; 20 ; 60) : 100 ml ; solution (1) : dissolve 0.1 g of sample in 5 ml
of 2 M ammonia* ; solution (2) : dilute 1 ml of soln. (1) to 50 ml with water ;
solution (3) : dilute 5 ml of solution (2) to 20 ml with water ; Solution (4) :
dissolve 10 mg of glutamic acid EPCRS in sufficient water to produce 50 ml ;
solution (5) dissolve 10 mg of glutamic acid EPCRS and 10 mg of aspartic acid
EPCRS in sufficient water to produce 25 ml ; ninhydrin solution (0.2% w/v
solution of ninhydrin in a mixture of 95 vols. of butan-1-ol and 5 vols of 2 M
acetic acid**) : 50 ml ;
Procedure : Apply separately to the silica
gel G coated plates 5 μ l of each of sols (1), (2), (3), (4) and (5) and dry the
TLC plates in a current of air for 15 minutes before commencing development.
Carry out the development using the above mentioned mobile-phase as usual.
After removal of the plate, allow it to dry in air, spray with ninhydrin
solution and heat at 100° to 105 °C for 15 minutes.
Observations
·
Any secondary spot in the chromatogram obtained with
soln. (1) is less intense than the spot obtained with soln. (3).
·
The test is not valid unless the chromatogram obtained
with soln. (5) show two distinctly sepa-rated spots.
5.1. Congnate Assays
The assay of leucine-an amino acid official in BP (1993)
may also be carried out by adopting a similar procedure using the same
adsorbent and mobile-phase but different solution from (1) to (5).
Related Topics