Chapter: Pharmaceutical Drug Analysis: Thin Layer Chromatography (TLC)
Chemical Reactions on Thin Layer Chromatography (TLC) Plates
CHEMICAL REACTIONS ON TLC PLATES
Glass being an inert material used for TLC-plates renders
it ideal for utilization with strong corrosive reagents.
Miller and Kirchner* in 1953, were the pioneer in
originating and developing the novel ideal of per-forming chemical unit-process
reactions directly on TLC-plates. The two
major steps involved in achieving this objective are, namely :
(a) Sample is
spotted on a TLC plate in the usual manner and subsequently covered with a
specific reagent, and
(b) Soonafter
the reaction is completed, the TLC plate is developed using an appropriate
solvent thereby separating the products of the reaction.
In actual practice, the resulting Rf value of the
original compound together with the chromatographic results of the reaction are
usually good enough to identify a compound accurately and precisely.
Example : (i) Citral reacts with 30% H2O2 in
the presence of UV-light for a duration 10 minutes and undergoes catalytic
oxidation to yield geranic acid as shown below :
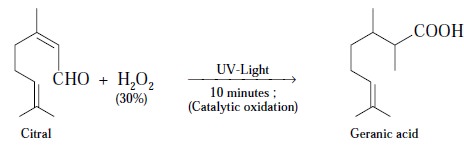
(ii) Citral undergoes
reduction in the presence of 10% w/v solution of LiAlH4 in ether to
produce geraniol as represented in the following reaction :

Exactly in the same manner, a number of other chemical
unit-process reactions may be accomplished on TLC plates as stated here briefly
:
(a) Dehydration :
Sample spot of terpene
alcohols e.g., linalol, be converted
to hydrocarbons by adding a drop of conc. H2SO4 as shown
below :
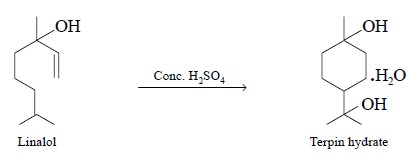
Consequently, the TLC plate is developed with hexane and
since oxygenated compounds, do not move in hexane (i.e., stay-back), only the hydrocarbons thus generated move away
from the specific-reaction zone.
(b) Bromination :
Cargill* in 1962,
separated cholestanol from cholesterol by TLC. The mixture is spotted on a TLC
plate and reacted with a soln. of Br2 (0.1% w/v in CHCl3),
taking care that its quantity must be 2 to 3 times the weight of the sample
mixture. Development in a solvent system consisting of benzene and ethyl
acetate (2 : 1) would result in a clear distinction of cholestanol and reaction
products of cholesterol with Br2.
(c) Enzymatic Reaction :
Randerath and
Randerath** in 1964, demonstrated an enzymatic reaction directly on an
anion-exchange layer of cellulose impregnated with polyethylene imine. A
buffered solution of phosphodiesterase is applied to the sample spot of
cytidine dipohosphate glucose, which is subsequently covered with paraffin and
allowed to stand for 45-60 minutes at 23°C. Chromatography of the resulting
degradation products gives rise to cytidine 5-monophosphate and glucose
1-phosphate.
(d) Esterification :
Benneth and
Heftmann*** in 1962, showed that it was feasible to esterify the C-3, hydroxy
steroids directly on TLC plates by means of tri-fluoroacetic anhydride. After
treating the compounds with the anhydride, it is absolutely necessary to dry
the pate in the hood for several minutes so as to get rid of the trifluoroacetic
acid that is produced as a by-product.
Related Topics