Chapter: Pharmaceutical Drug Analysis: Thin Layer Chromatography (TLC)
Choice Of Solvent System in Thin Layer Chromatography (TLC)
CHOICE OF SOLVENT SYSTEM IN TLC
The choice of solvent or a mixture of solvents used in
TLC is solely guided by two important factors : (a) the nature of the constituent to be separated i.e., whether it is polar or non-polar ;
and (b) the nature of the process
involved i.e., whether it is a case
of ‘adsorption’ or ‘partition chromatography’. It has been observed that the
rate of migration of a substance on a given adsorbent depends upon the solvent
used ; therefore, the latter may be arranged in order of the elutive power,
usually termed as the elutropic series
as shown in the following Table 28.1.
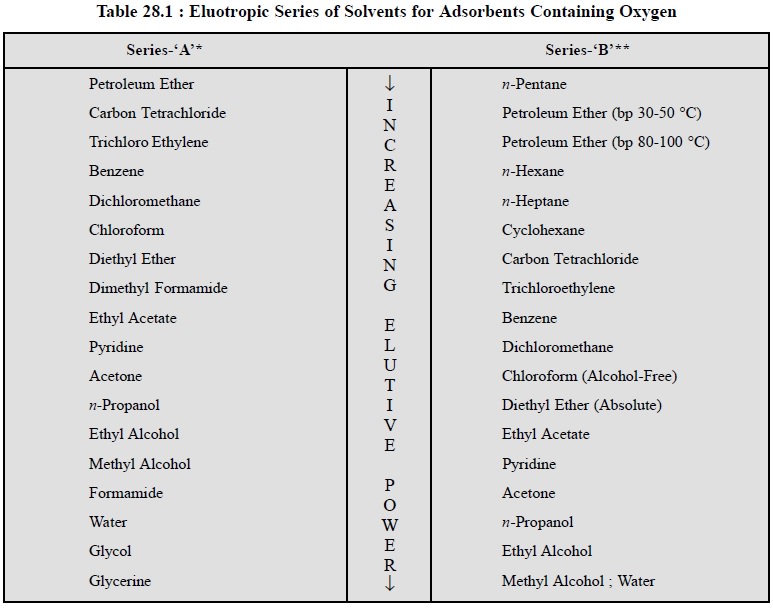
Note : (i) These series are not always valid in
precisely the same order for all substances,
(ii) These series may be regarded as good guides for selecting a
specific solvent only, and
(iii) These series are good for hydrophyllic adsorbents and not for
hydrophobic ones e.g., charcoals.
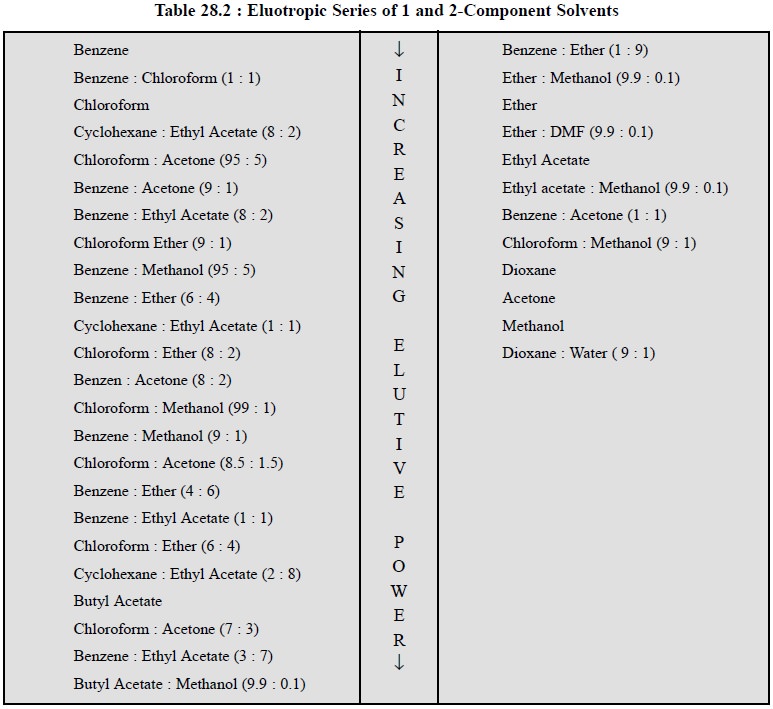
From actual experimental results it has been established
beyond any reasonable doubt that the mix-tures of two or three solvents of
different polarity mostly offer distinct and much improved separation as
compared to chemically homogeneous solvents. Table 28.2 records the elutropic
series of one and two com-ponent solvents.
Related Topics