Chapter: Pharmaceutical Drug Analysis: Thin Layer Chromatography (TLC)
Development of Thin Layers - Thin Layer Chromatography (TLC)
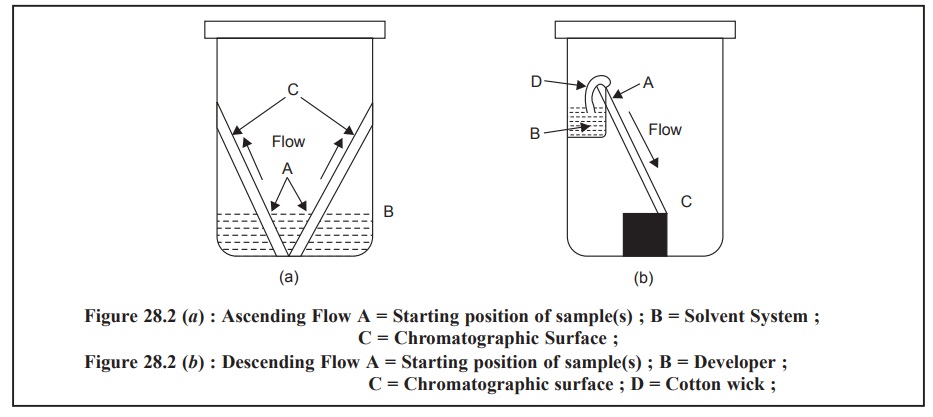
DEVELOPMENT OF THIN LAYERS
The spotted TLC plates, after evaporation of the sample
solvent, is placed in a closed chamber saturated with vapours of the developing
solvent(s). One end of the plate is then wetted with the developer by means of
either ‘ascending-technique or the ‘descending-technique’ as shown in Figure
28.2 (a), (b). After the devel-oper has traversed one-half to two-thirds the
total length of the TLC plate, the latter is removed from the chamber,
air-dried and the positions of the components are located by any of several
methods.
There are three
major factors which essentially govern the ‘development of thin-layers’, namely
:
(i)
Equilibration of the chamber (or chamber-saturation),
(ii) Protection
against oxidation (temperature and light), and
(ii) Visualization.
1. Equilibration of the Chamber
The equilibration of the chamber or chamber-saturation is
a vital factor to obtain reproducible Rf values. It may be achieved by allowing
the solvent system to remain in the chamber for at least 1 to 2 hours so that
the vapours of the solvent(s) would pre-saturate the latter adequately. This is
done to obtain distinct separation of constituents, uniform solvent from and
prevent evaporation of the solvent on TLC-plates.
2. Protection against Oxidation
Both temperature and light augments oxidation and,
therefore, ideally the following experimental parameters must be observed to
obtain the best development of thin-layers, viz.,
Temperature : 18-23°C, and
Light : Diffused daylight both natural and artificial,
However, direct sunlight (UV) or drought may give rise to
‘oblique formation’ of the solvent front.
3. Visualization
As a result of both intensive as well as extensive
research a number of organic and inorganic substances have been identified that
positively demonstrate an ‘improved
visualization’. Such substances are termed collectively as ‘fluorescent indicators’.
Examples : Barium diphenylamine
sulphonate ; 2,7-dichlorofluorescein ; Fluorescein (0.2% w/v in Ethanol)
; Morin (0.1% w/v in Ethanol) ; Sodium fluorescinate (0.4% w/v in water) ;
Rhodamine B ; Zinc Silicate ; Calcium silicate ; Methylumbelliferone (or
7-hydroxy-4-methyl coumarin).
Related Topics