Chapter: Organic Chemistry: Alkyl halides
Preparation and physical properties of alkyl halides
PREPARATION AND PHYSICAL PROPERTIES OF ALKYL
HALIDES
Key Notes
Preparation
Alkenes
are converted to alkyl halides by reaction with hydrogen halides. Treatment
with halogens results in dihaloalkanes. Tertiary alcohols can be converted to
alkyl halides on treatment with hydrogen halides, whereas pri-mary and
secondary alcohols are best converted by using thionyl chloride or phosphorus
tribromide.
Structure
Alkyl
halides consist of an alkyl group linked to a halogen. The carbon linked to the
halogen is sp3 hybridized
and tetrahedral. The carbon–halogen bond length increases and the bond strength
decreases as the halogen increases in size.
Bonding
The
C–halogen bond (C–X) is a polar σ bond where the halogen is slightly negative
and the carbon is slightly positive. Intermolecular bonding is by weak van der
Waals interactions.
Properties
Alkyl
halides have a dipole moment. They are poorly soluble in water, but dissolve in
organic solvents. They react as electrophiles at the carbon center.
Reactions
Alkyl
halides undergo nucleophilic substitution reactions and elimination reactions.
Spectroscopic analysis
The
presence of a halogen atom can be shown by IR spectroscopy (C–X stretching
absorptions) as well as by mass spectroscopy. The latter shows a characteristic
pattern of peaks for the molecular ion that matches the number and ratio of
naturally occurring isotopes of the halogen. Elemental analysis also
demonstrates the presence of halogens.
Preparation
Alkenes can be treated with hydrogen halides
(HCl, HBr, and HI) or halogens (Cl2 and Br2) to
give alkyl halides
and dihaloalkanes respectively
. Anextremely useful method of preparing alkyl halides is to treat an
alcohol with ahydrogen halide (HX = HCl, HBr, or HI). The reaction works best
for tertiaryalcohols. Primary and secondary alcohols can be converted to
alkylhalides more effectively by treating them with thionyl chloride (SOCl2)
or phosphorus tribromide (PBr3). The conditions are less acidic and
less likely to cause acid-catalyzed rearrangements.
Structure
Alkyl halides consist of an alkyl group linked
to a halogen atom (F, Cl, Br, or I) by a single (σ) bond. The carbon atom linked to the halogen atom is sp3 hybridized and has a tetrahedral geometry
with bond angles of approximately 109 . The carbon–halogen bond length
increases with the size of the halogen atom and this is associated with a
decrease in bond strength. For example, C–F bonds are shorter and stronger than
C–Cl bonds.
Bonding
The carbon–halogen bond (referred to as C–X
from here on) is a bond. The bond is polar since the halogen atom is more
electronegative than carbon, resulting in The halogen being slightly negative
and the carbon being slightly positive. Intermolecular hydrogen bonding or
ionic bonding is not possible between alkyl halide molecules and the major
intermolecular bonding force consists of weak van der Waals interactions.
Properties
The polar C–X bond means that alkyl halides
have a substantial dipole moment. Alkyl halides are poorly soluble in water,
but are soluble in organic solvents. They have boiling points which are similar
to alkanes of comparable molecular weight. The polarity also means that the
carbon is an electrophilic center and the halogen is a nucleophilic center.
Halogens are extremely weak nucleophilic centers and therefore, alkyl halides
are more likely to react as electrophiles at the carbon center.
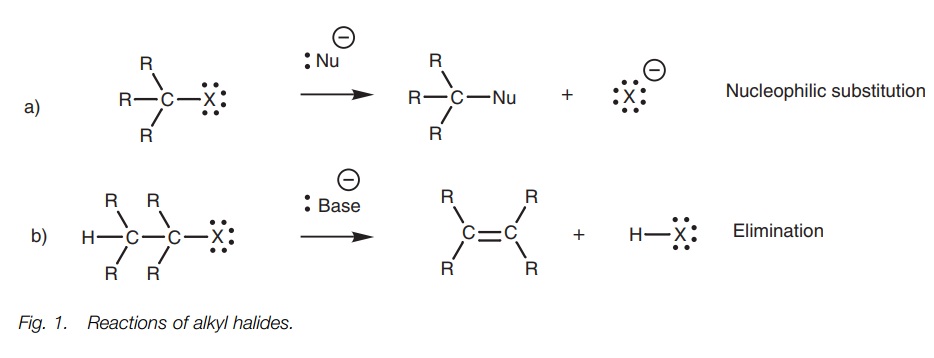
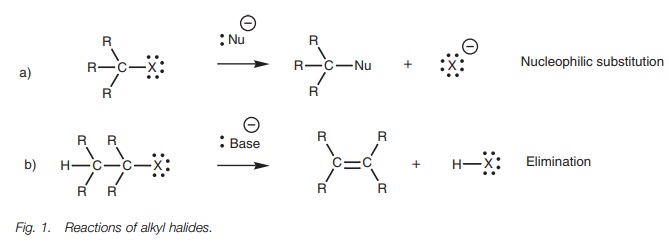
Reactions
The major reactions undergone by alkyl halides
are (a) nucleophilic substitution where an attacking nucleophile replaces the
halogen (Fig. 1a), and (b)
elimination where the alkyl halide loses HX and is converted to an alkene (Fig. 1b).
Spectroscopic analysis
The IR spectra of alkyl halides usually show
strong C–X stretching absorptions. The position of these absorptions depends on
the halogen involved i.e. the absorptions for C–F, C–Cl, C–Br and C–I occur in
the regions 1400–1000, 800–600, 750–500 and 500 cm−1 respectively.
The presence of a halogen can sometimes be
implicated by the chemical shifts of neighboring groups in the nmr spectra. For
example the chemical shifts in the 1H nmr spectrum for CH2I,
CH2Br and CH2Cl are 3.2, 3.5 and 3.6
respectively.
Good evidence for the presence of a halogen
comes from elemental analysis and mass spectroscopy. In the latter, there are
characteristic peak patterns associated with particular halogens as a result of
the natural abundance of various isotopes. For example, bromine has two
naturally occurring isotopes of 79 and 81 that occur in a ratio of 1 : 1. This
means that two peaks of equal intensity will be present for any organic
compound containing bromine. For example, the mass spectrum for ethyl bromide
has two peaks of equal intensity at m/e 108 and 110 for the molec-ular ions 12C21H579Br
and 12C21H581Br
respectively.
In contrast, chlorine occurs naturally as two
isotopes (35Cl and 37Cl ) in the ratio 3 : 1. This means
that the mass spectrum of a compound containing a chlorine atom will have two
peaks for the molecular ion. These peaks will be two mass units apart with an
intensity ratio of 3 : 1.
Related Topics